



Licorice, is an herbal plant renowned for its distinct sweet flavor. It is called "GAN CAO" in Chinese and is derived from the root or stem of Glycyrrhiza glabra (G. glabra). Its primary active components consist of triterpenes and flavonoids.
Liquiritigenin is a dihydroxyflavone, one of the main bioactive components extracted from licorice. It has also been applied in a variety of fields, such as healthare, cosmetic, antibiotic, plant physiology, and quality indicator component of licorice root. Furthermore, it also serves as an intermediate for the synthesis of drugs with anti-diabetic, anti-tuberculosis and other properties, offering significant potential and vast application prospects in the healthcare field.1
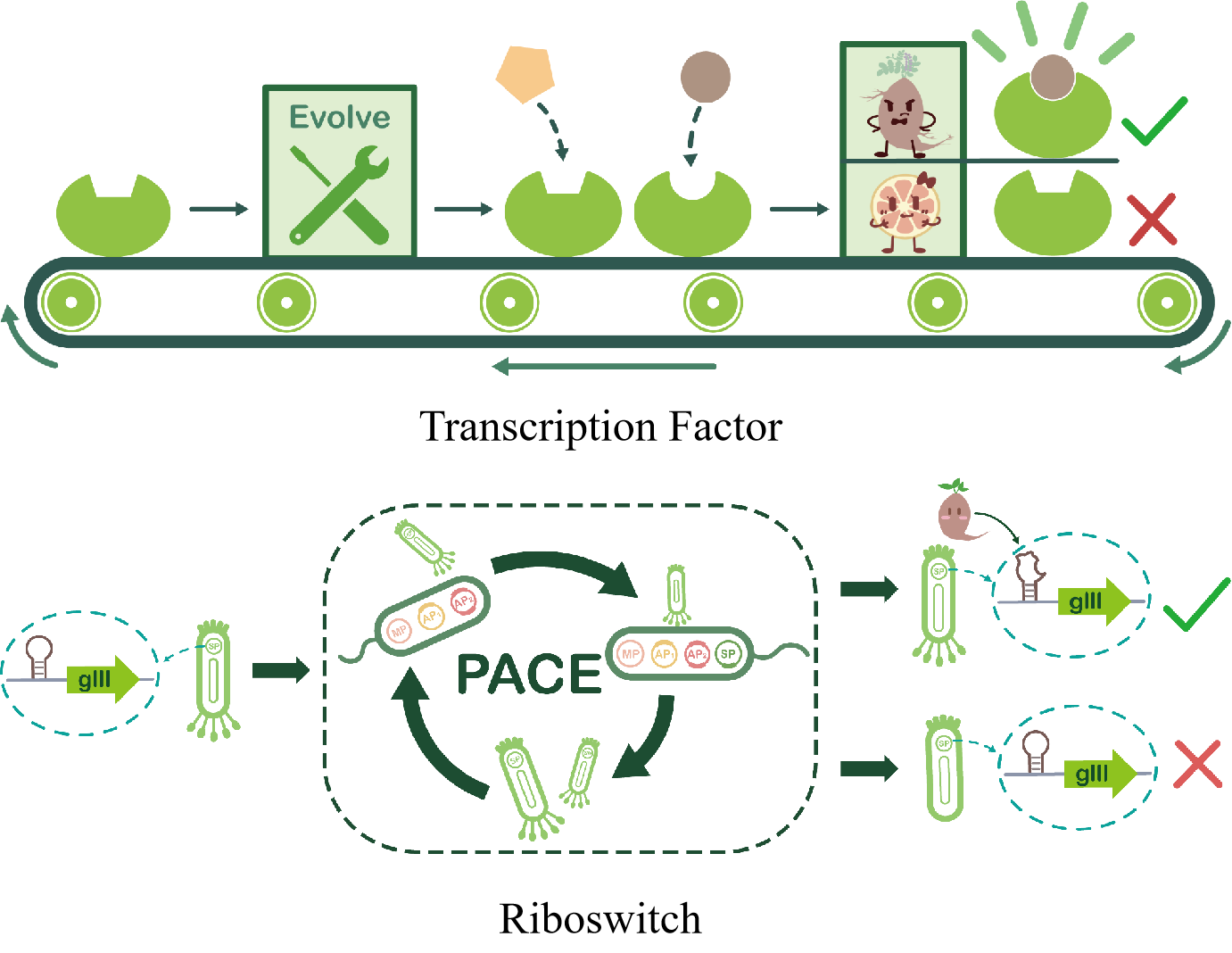
However, the production of liquiritigenin still relies on low-yield plant extraction and chemical synthesis currently. Here, we developed biosensors that could identify liquiritigenin specifically based on allosteric transcription factors and riboswitches, enabling intracellular detection of liquiritigenin synthesis and screening of liquiritigenin high yielding strains, thus providing a solution for high-yielding biosynthesis of liquiritigenin.
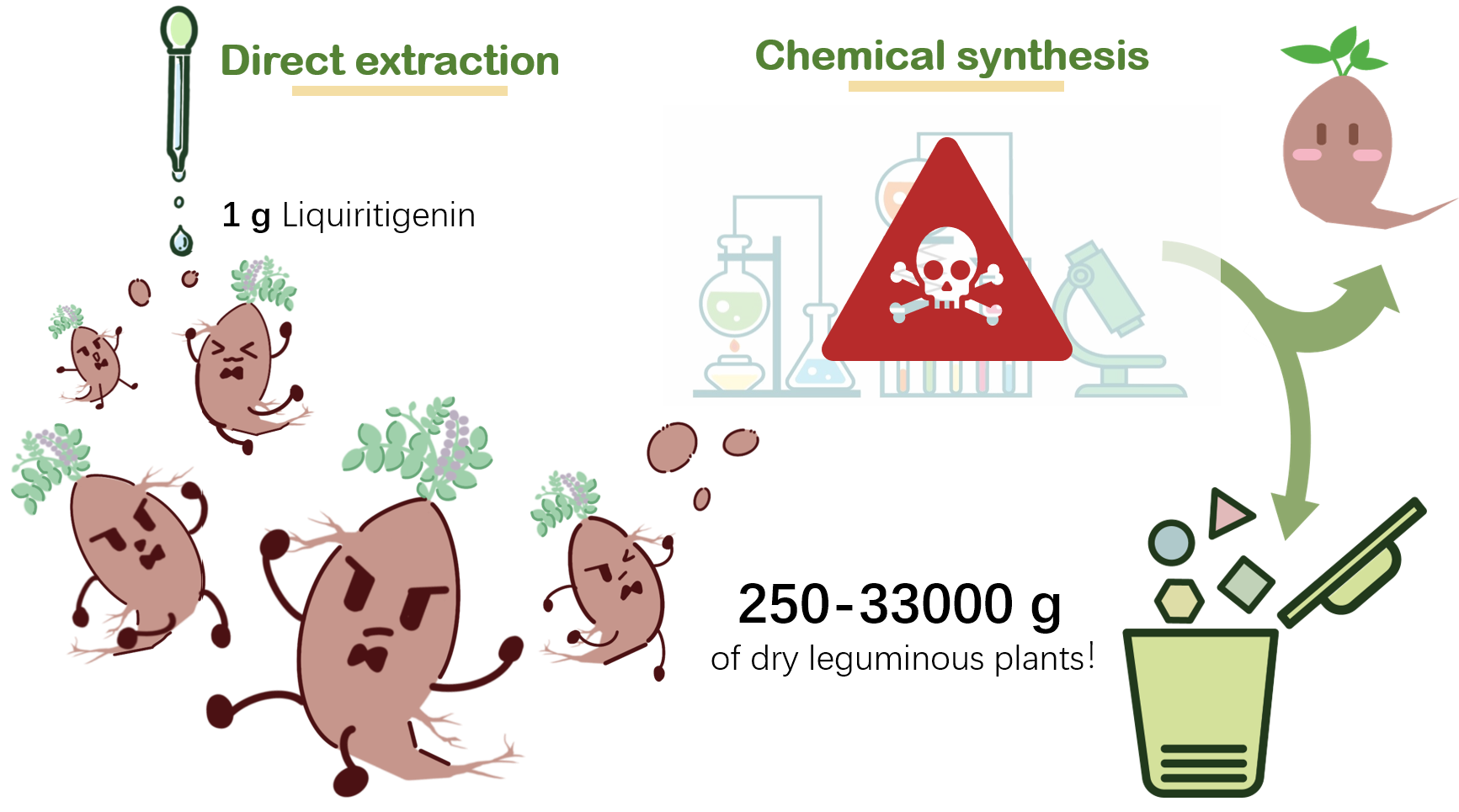
The prevalent methods of liquiritigenin production, such as plant extraction and chemical synthesis, have their inherent limitations. The former is constrained by long growth cycles, high costs, and low yields (approximately 1 gram from 250-33,000 g of dry leguminous plants). The latter involves toxic solvents, extreme reaction conditions, and complex molecular modifications, making it unsuitable for industrial production and harmful to the environment.
Microbial production is an attractive candidate for flavonoid production with advantages like low energy consumption, minimal waste, environmental friendliness.2,3Although significant research has explored the heterologous synthesis of 5-deoxy(iso) flavonoids in microbial hosts, the yield remains relatively low.4,5A tool is needed for high-yield strain screening to increase the yield of liquiritigenin and monitoring liquiritigenin in production.

As a real-time, highly sensitive concentration detection element, a biosensor is just such a suitable tool. But biosensors that specifically and efficiently recognize liquiritigenin are not yet available. However, naringenin, a structural analog of liquiritigenin, can be efficiently identified by artificial biosensors in previous research, including transcription factors and riboswitches.6 Based on this, we selected allosteric transcription repressor factor FdeR and a riboswitch that identify naringenin as starting points.

Therefore, we start with allosteric transcription repressor factor FdeR and riboswitches, and try to use error-prone PCR, site-directed mutation and phage-assisted continuous evolution (PACE) strategies to enable them to specifically recognize liquiritigenin
1. M. Ramalingam, H. Kim Y. Lee et al, Phytochemical and Pharmacological Role of Liquiritigenin and Isoliquiritigenin From Radix Glycyrrhizae in Human Health and Disease Models. Front Aging Neurosci 10, 348, (2018).
DOI: http://doi.org/10.3389/fnagi.2018.00348
2. J.A. Chemler, Y. Yan M.A. Koffas, Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb Cell Fact 5, 20, (2006).
DOI: http://doi.org/10.1186/1475-2859-5-20
3. Y. Katsuyama, M. Matsuzawa N. Funa et al, Production of curcuminoids by Escherichia coli carrying an artificial biosynthesis pathway. Microbiology (Reading) 154, 2620-2628, (2008).
DOI: http://doi.org/10.1099/mic.0.2008/018721-0
4. J. Li, F. Xu D. Ji et al, Diversion of metabolic flux towards 5-deoxy(iso)flavonoid production via enzyme self-assembly in Escherichia coli. Metabolic Engineering Communications13, e00185, (2021).
DOI: https://doi.org/10.1016/j.mec.2021.e00185
5. S.G. Stahlhut, S. Siedler S. Malla et al, Assembly of a novel biosynthetic pathway for production of the plant flavonoid fisetin in Escherichia coli. Metabolic engineering 31, 84-93, (2015).
DOI: https://doi.org/10.1016/j.ymben.2015.07.002
6. S. Jang, S. Jang Y. Xiu et al, Development of Artificial Riboswitches for Monitoring of Naringenin In Vivo. ACS Synth Biol 6, 2077-2085, (2017).
DOI: http://doi.org/10.1021/acssynbio.7b00128