
Overview
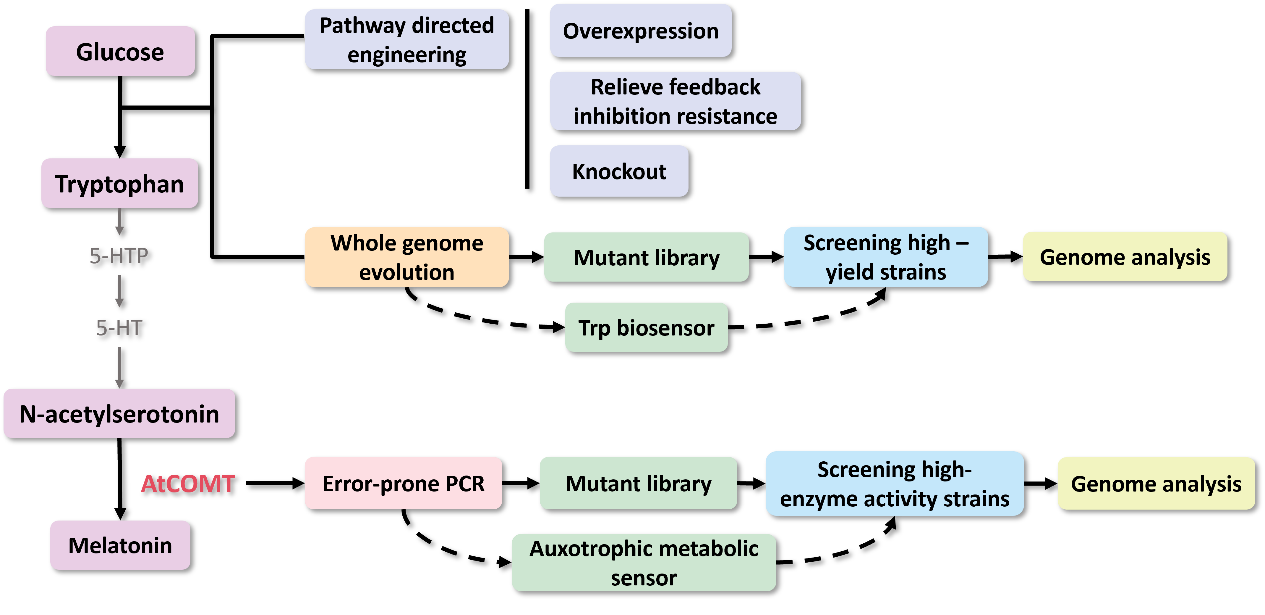
Module 1: Efficient synthesis of L-Tryptophan (L-Trp)
Part 1: Rational Metabolic Engineering
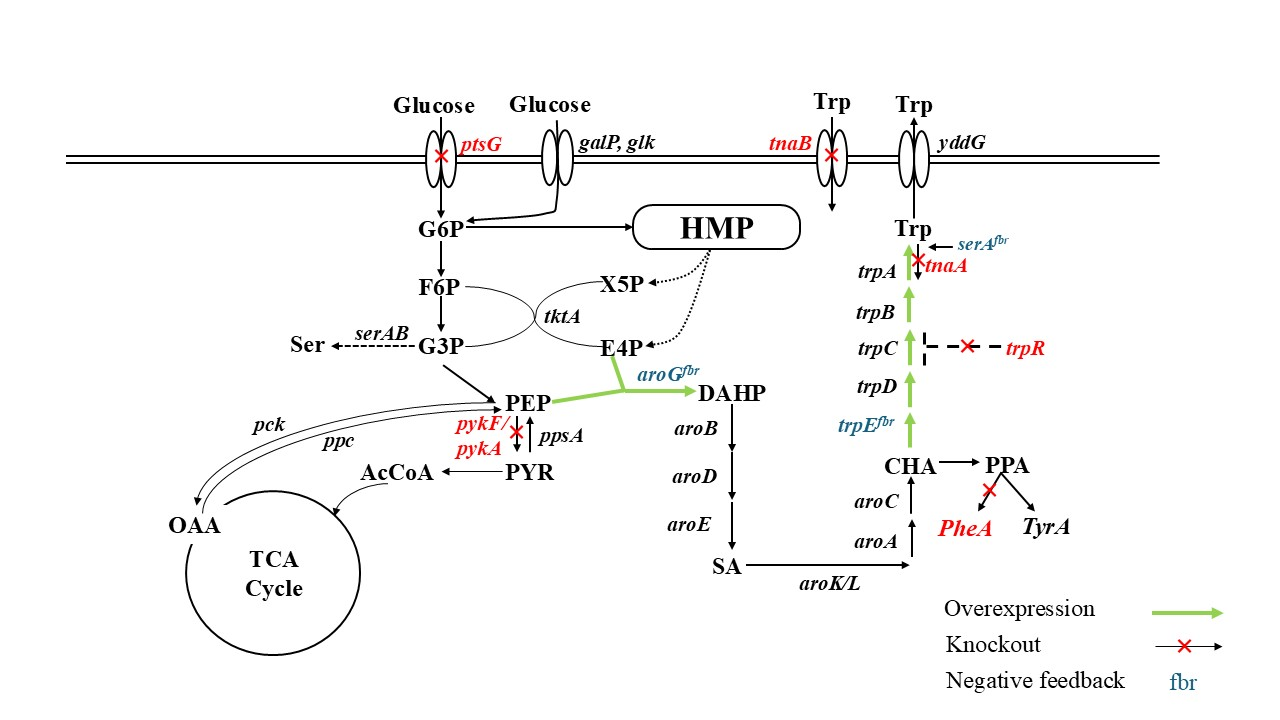
To improve tryptophan yield, we engineered its metabolic pathway by overexpressing biosynthetic genes, mutating feedback-resistant alleles, and eliminating branch pathway genes. Firstly, we designed overexpression plasmids pTRP1 and pTRP2 with feedback-resistant variants carrying the trpES40F mutation, rendering anthranilate synthase insensitive to inhibition; pTRP3 further integrates aroGfbr and serAfbr for enhanced supply of precursors DAHP and L-serine.
For flux redirection, we pruned competing pathways draining key intermediates (PEP, E4P, chorismate): ΔptsG boosts PEP by modulating glucose uptake1; ΔpykF/ΔpykA blocks pyruvate kinase-mediated PEP loss2; and ΔpheA diverts chorismate from phenylalanine synthesis3. Additionally, we knocked out trpR to alleviate its feedback inhibition on the trpEDCBA operon, and deleted tnaAB to prevent the reuptake of extracellular tryptophan4.
Part 2: Directed Evolution for Enhanced L-Trp Yield
Under low L-Trp conditions, decoding release factor 2 (RF2) specifically recognizes the UGA stop codon and promotes the cleavage of peptidyl-tRNAPro at the peptidyl transferase center (PTC) in the P-site of the ribosome. As a result, the ribosome rapidly dissociates from the nascent mRNA5.
The action of RF2 is not inhibited under these conditions, allowing the ribosome to disassemble after translating the tnaC open reading frame. This exposes the rut site, enabling Rho binding and resulting in Rho-dependent termination. Consequently, downstream genes mCherry (used for fluorescence) and Cmr (conferring chloramphenicol resistance) are not transcribed.
In contrast, under high L-Trp conditions, RF2 function is inhibited, leading to ribosomal stalling at the end of the tnaC open reading frame. This stalled ribosome physically masks the Rho binding site, thereby preventing termination and allowing transcription to proceed.


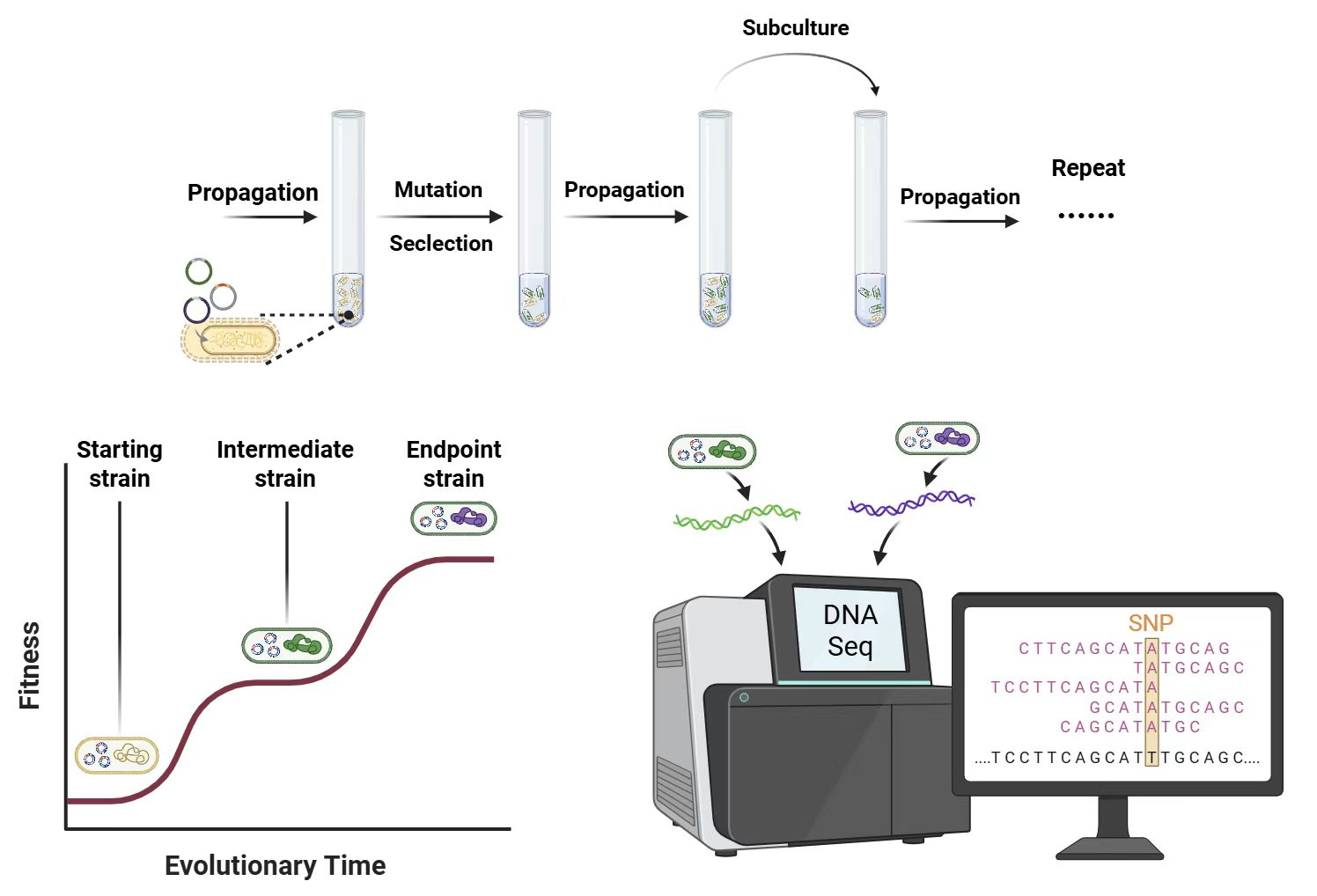
In our workflow, we integrated the MP6 plasmid into the optimized BW-RT chassis for arabinose-inducible, broad-spectrum genome mutagenesis6. Cells were co-transformed with the biosensor, then subjected to iterative rounds of chloramphenicol selection (12 mg/L), enriching populations for top performers. Then, the whole genome was analyzed using bioinformatics methods.
Module 2: Directed Evolution of AtCOMT Using a Whole-Cell Biosensor
Based on previous research, we designed a whole-cell biosensor by knocking out the cysE gene in Escherichia coli7. The resulting E. coli ΔcysE strain is unable to synthesize cysteine endogenously and cannot survive without external supplementation. We further introduced the cys3 and cys4 genes to establish a cysteine salvage pathway, in which S-adenosylmethionine (SAM) is utilized by AtCOMT and sequentially converted via homocysteine and cystathionine to ultimately synthesize cysteine. Consequently, bacterial growth directly depends on the enzymatic activity of AtCOMT.
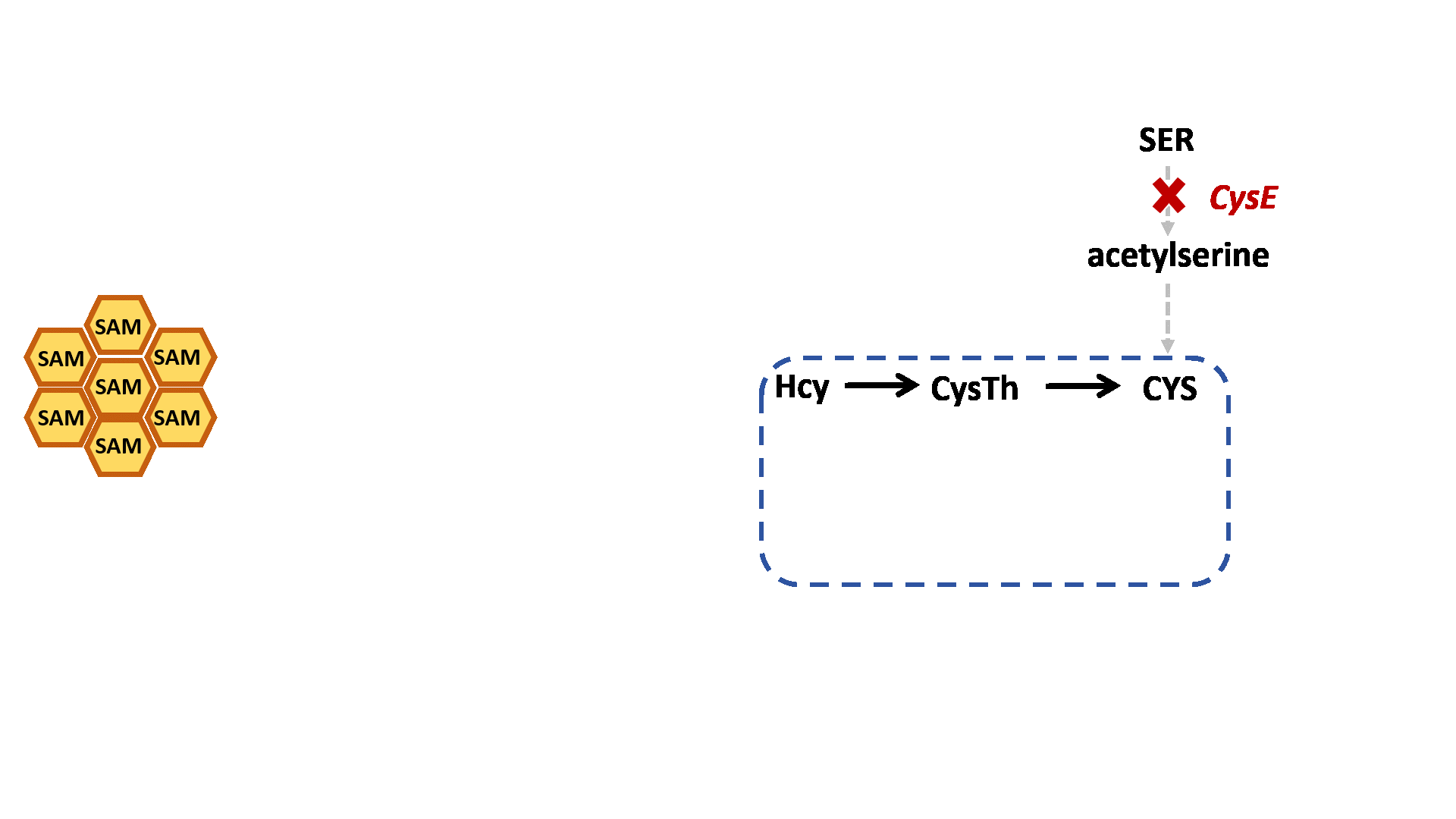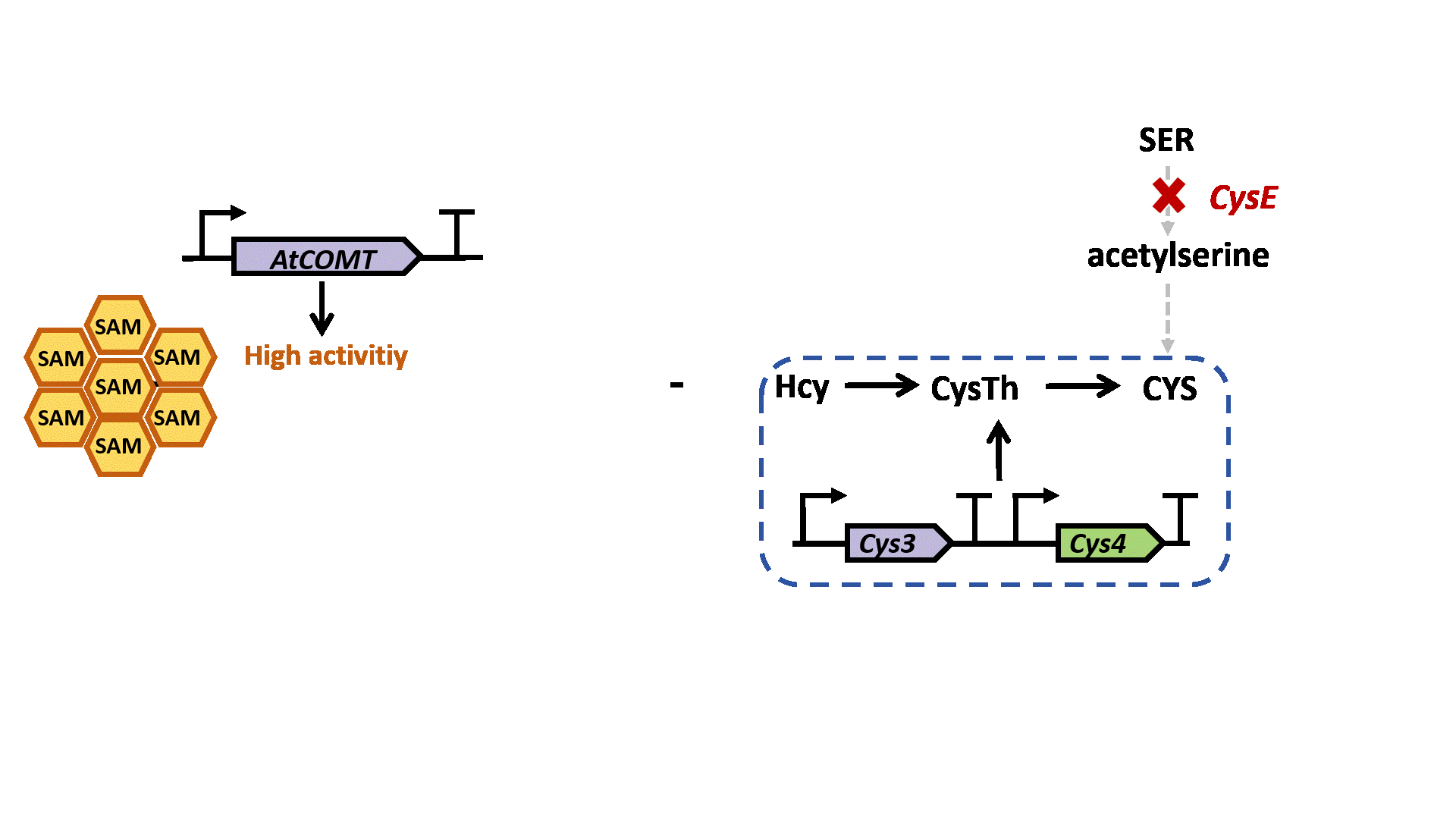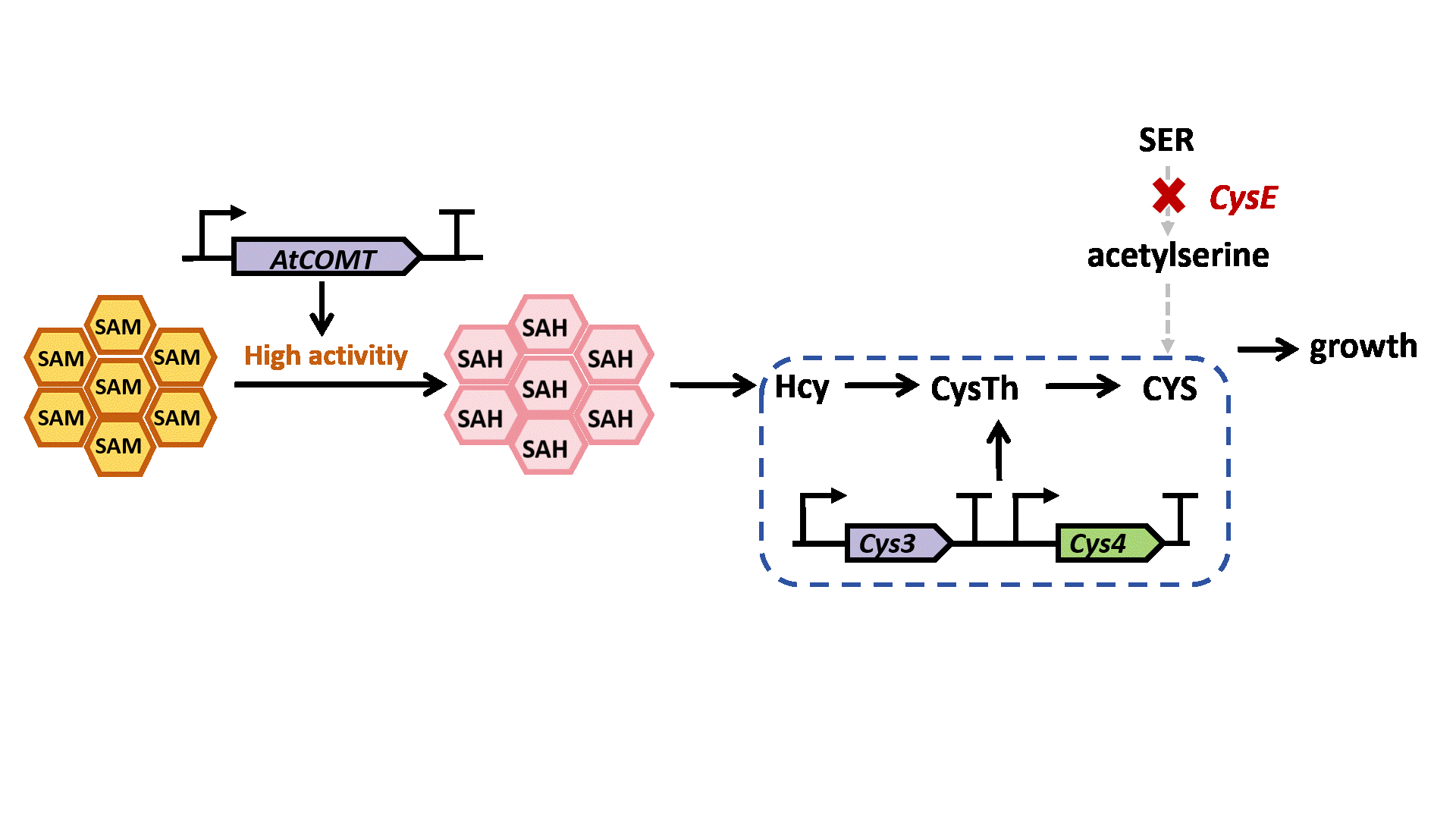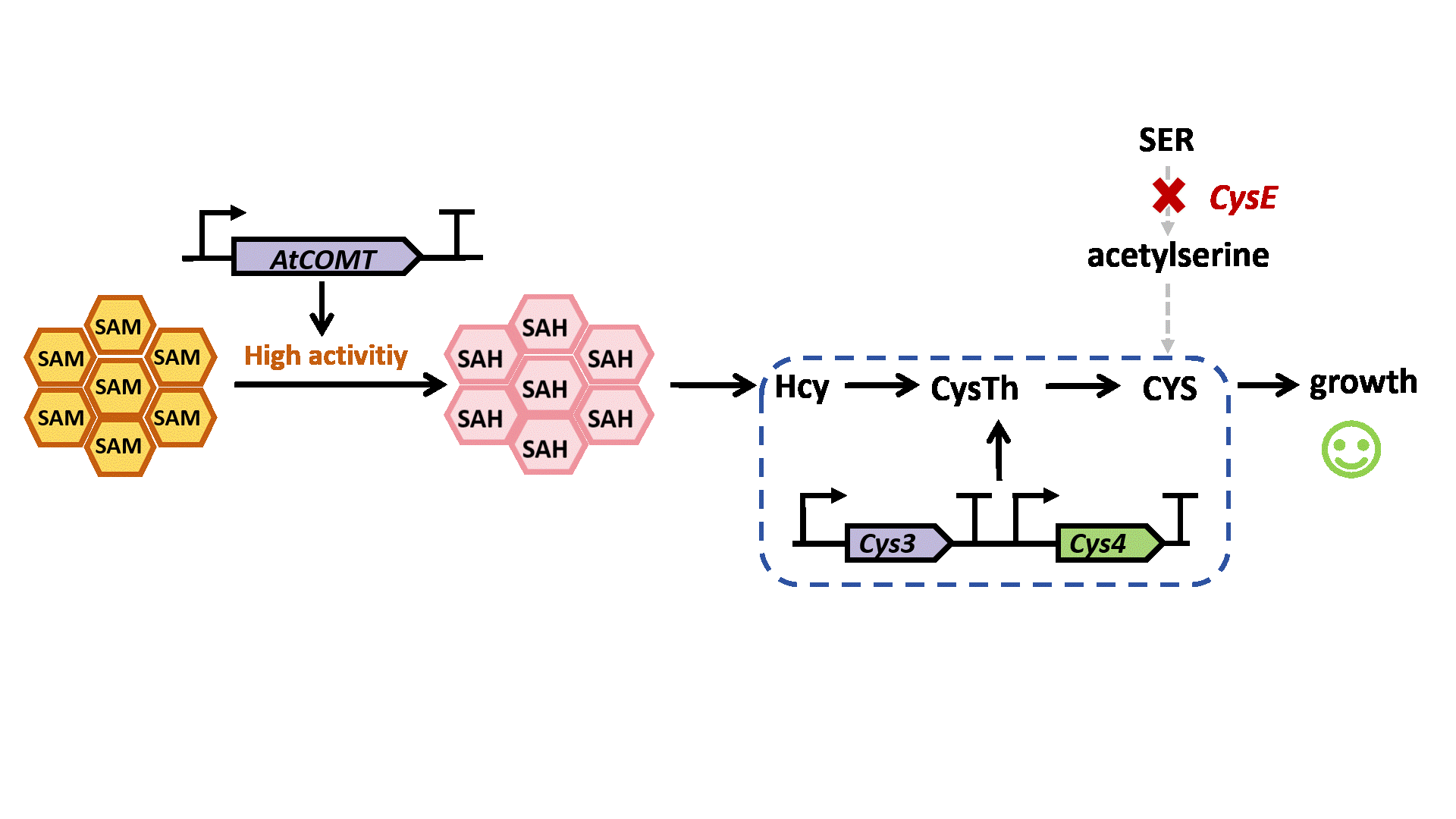
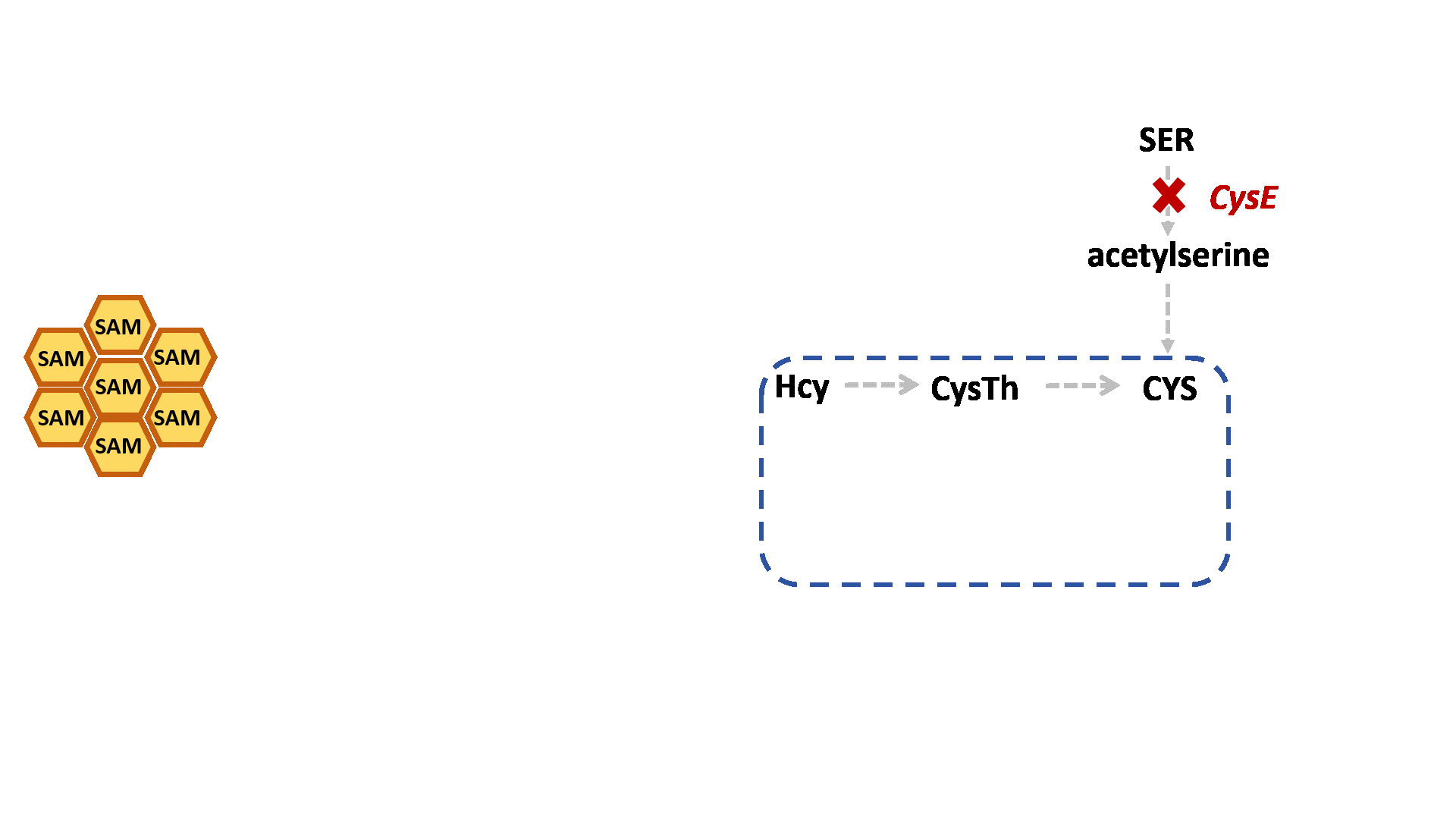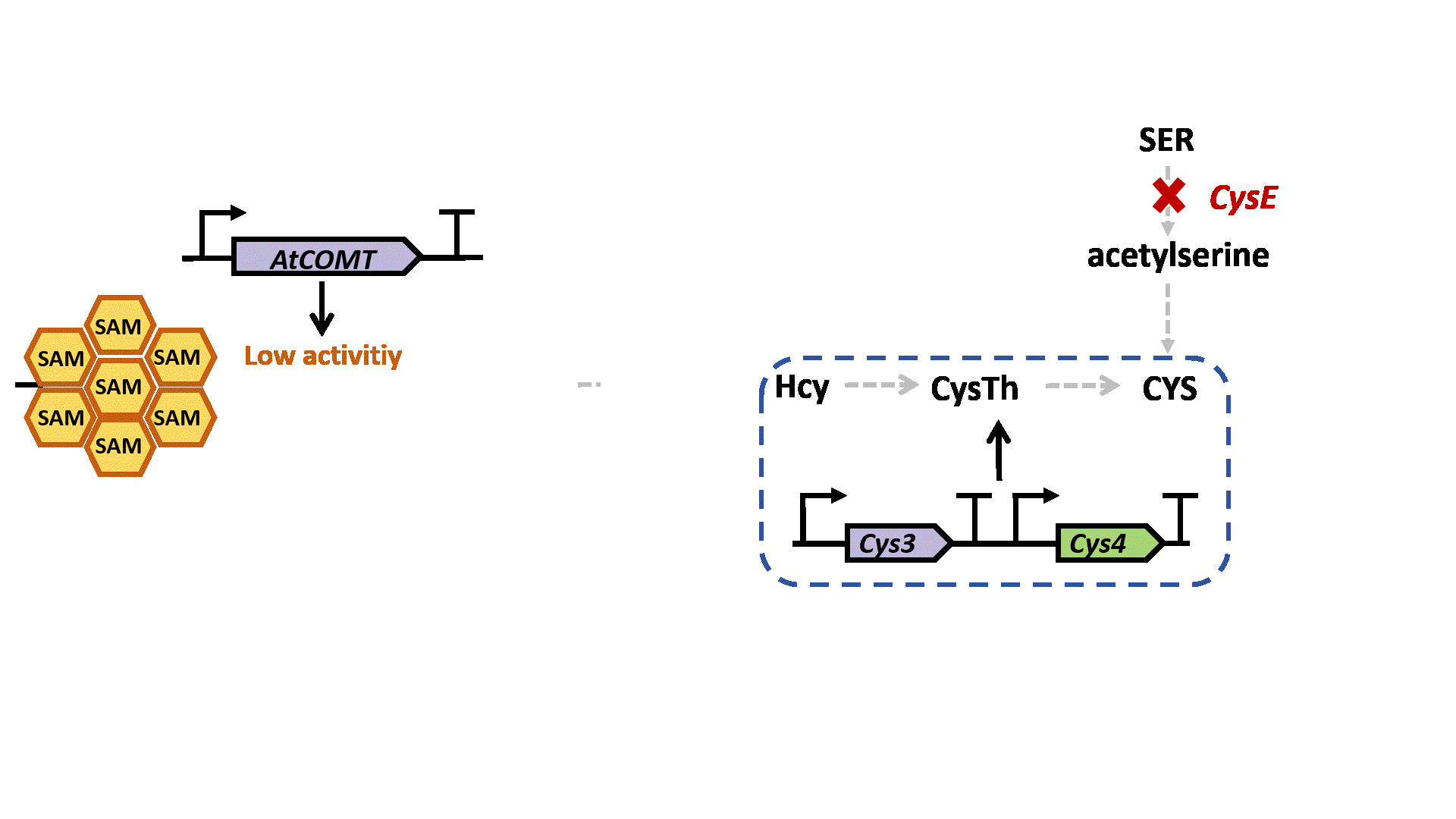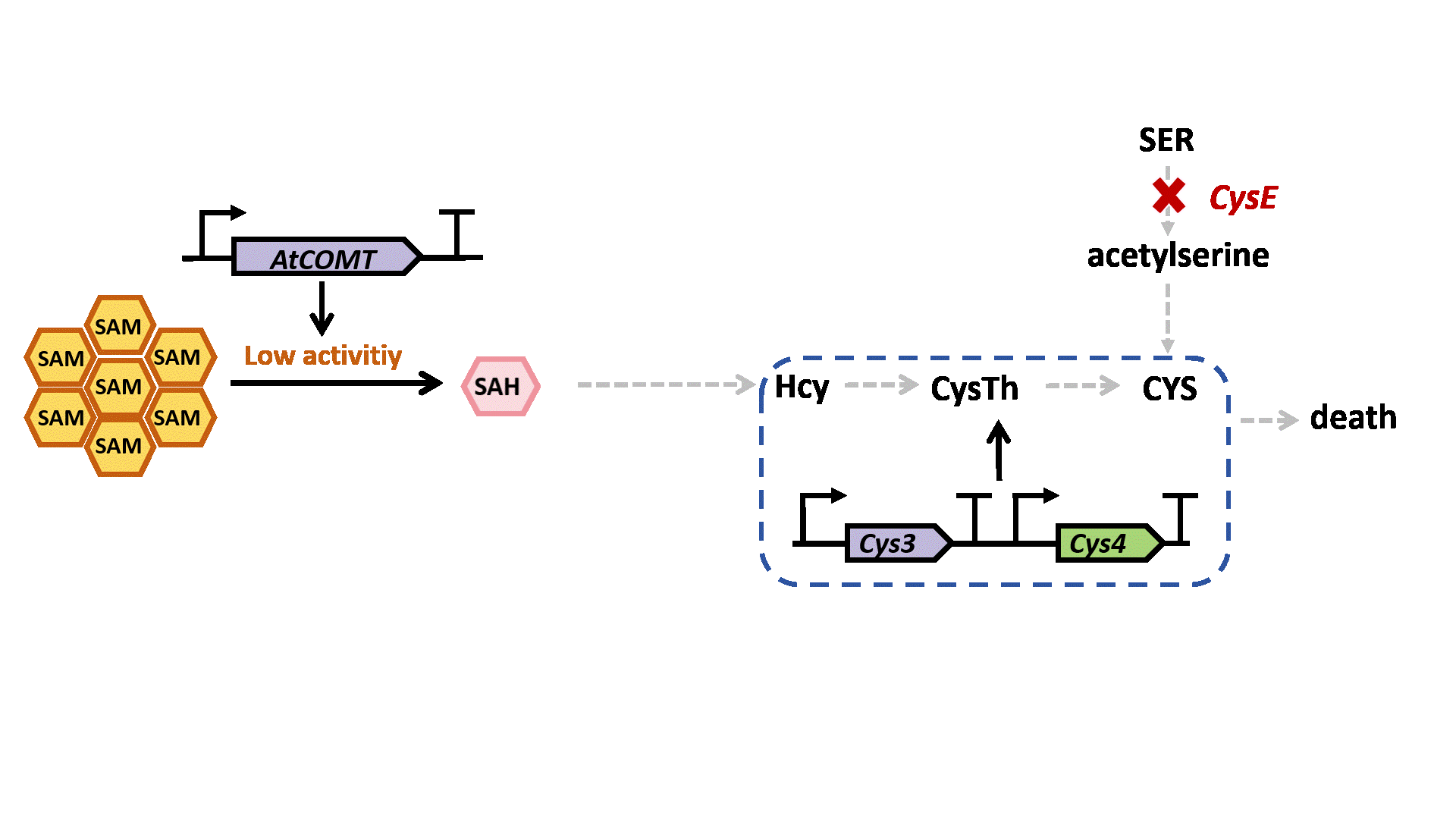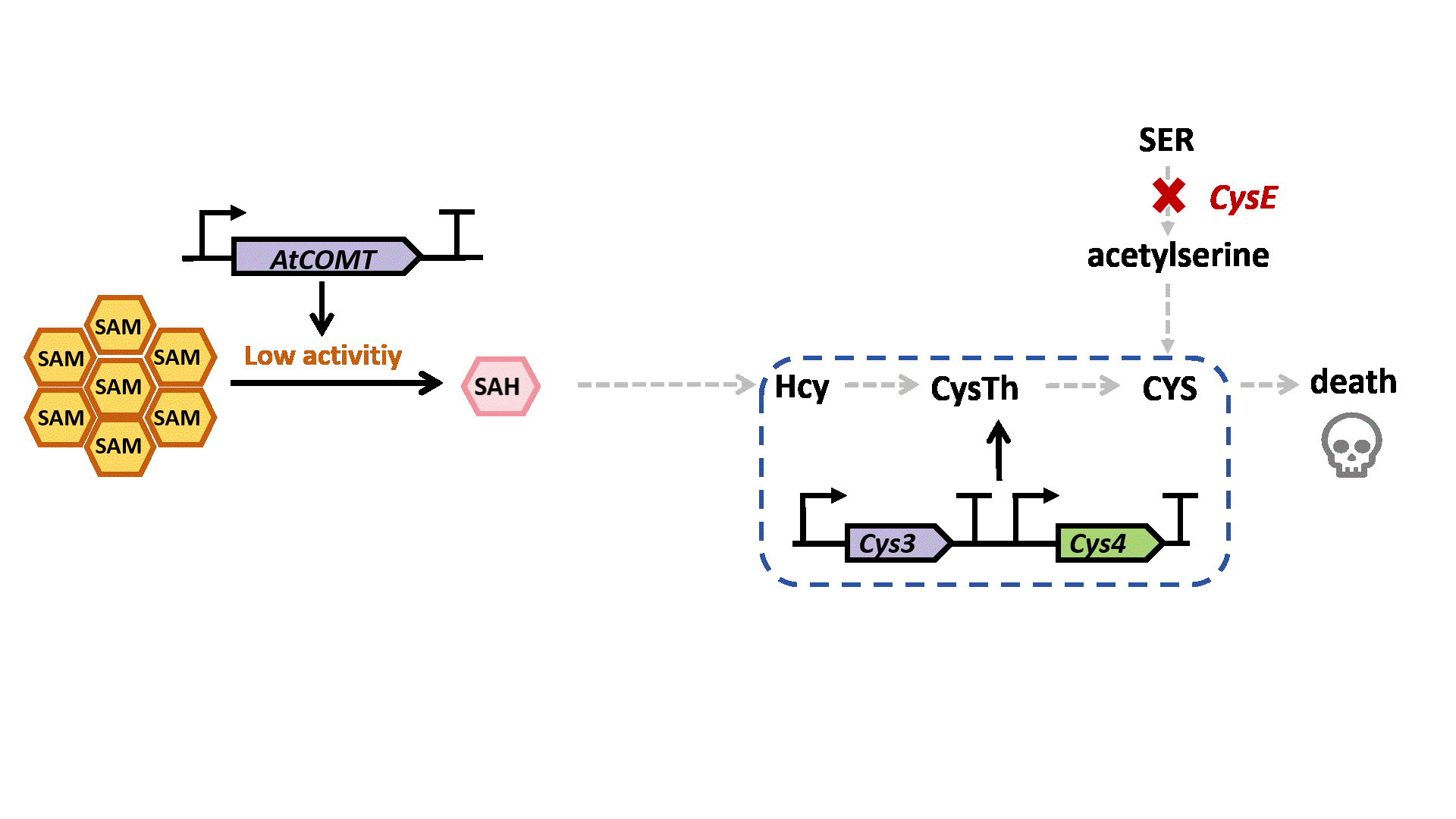
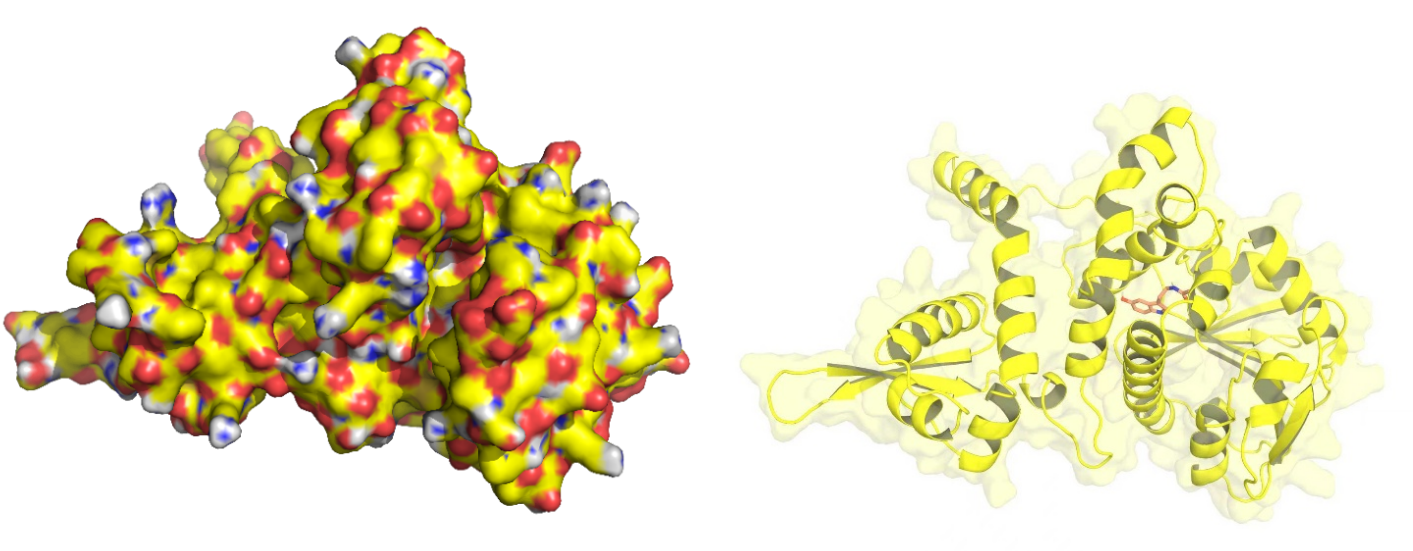
Upon generating a diverse library of AtCOMT mutants via error-prone PCR, we transformed them into E. coli and employed the biosensor for high-throughput screening. Subsequently, the enzymatic activity of AtCOMT in the selected monoclonal strains was quantitatively assessed using high-performance liquid chromatography (HPLC). Finally, to elucidate the mechanistic basis of the interaction between AtCOMT and its substrate N-Acetylserotonin (NAS) at the atomic level, we performed molecular docking simulations to characterize their binding mode.
References
- Gu, P., Yang, F., Kang, J., Wang, Q., & Qi, Q. (2012). One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of L-tryptophan in Escherichia coli. Microbial Cell Factories, 11(1), 30. DOI: https://doi.org/10.1186/1475-2859-11-30
- Liu, L., Chen, S., & Wu, J. (2017). Phosphoenolpyruvate: glucose phosphotransferase system modification increases the conversion rate during L-tryptophan production in Escherichia coli. Journal of Industrial Microbiology and Biotechnology, 44(10), 1385-1395. DOI: https://doi.org/10.1007/s10295-017-1959-3
- Liu, X., Niu, H., Huang, Z., Li, Q., & Gu, P. (2020). Construction of a switchable synthetic Escherichia coli for aromatic amino acids by a tunable switch. Journal of Industrial Microbiology and Biotechnology, 47(2), 233-242. DOI: https://doi.org/10.1007/s10295-020-02262-y
- Hou, M., Gao, S., Wu, J. et al. Metabolic engineering of Escherichia coli to enhance L-tryptophan biosynthesis. Syst Microbiol and Biomanuf, 5, 622–634 (2025). DOI: https://doi.org/10.1007/s43393-025-00338-3
- van der Stel, A. X., Gordon, E. R., Sengupta, A. et al. Structural basis for the tryptophan sensitivity of TnaC-mediated ribosome stalling. Nat Commun, 12, 5340 (2021). DOI: https://doi.org/10.1038/s41467-021-25663-8
- Badran, A., Liu, D. Development of potent in vivo mutagenesis plasmids with broad mutational spectra. Nat Commun, 6, 8425 (2015). DOI: https://doi.org/10.1038/ncomms9425
- Khosla, C., Luo, H., Hansen, A. S. L., et al. Coupling S-adenosylmethionine–dependent methylation to growth: Design and uses. PLOS Biology, 17(3) (2019). DOI: https://doi.org/10.1371/journal.pbio.2007050
