
What is MT?
Melatonin (MT) is an evolutionarily conserved molecule that plays a key role in regulating circadian rhythms and sleep cycles in animals, plants, and microorganisms1,2. By synchronizing the biological clock, MT improves sleep quality, particularly in rhythm-disrupted or aging populations3. MT has significant global demand due to applications in sleep aids, anti-aging cosmetics, and animal husbandry.

Melatonin Production
Traditional MT production methods, including chemical synthesis and extraction from plant or animal sources, face environmental, safety, and economic limitations4,5. Microbial cell factories provide a sustainable and cost-effective alternative, reducing fermentation costs to approximately one-tenth of chemical synthesis6,7.
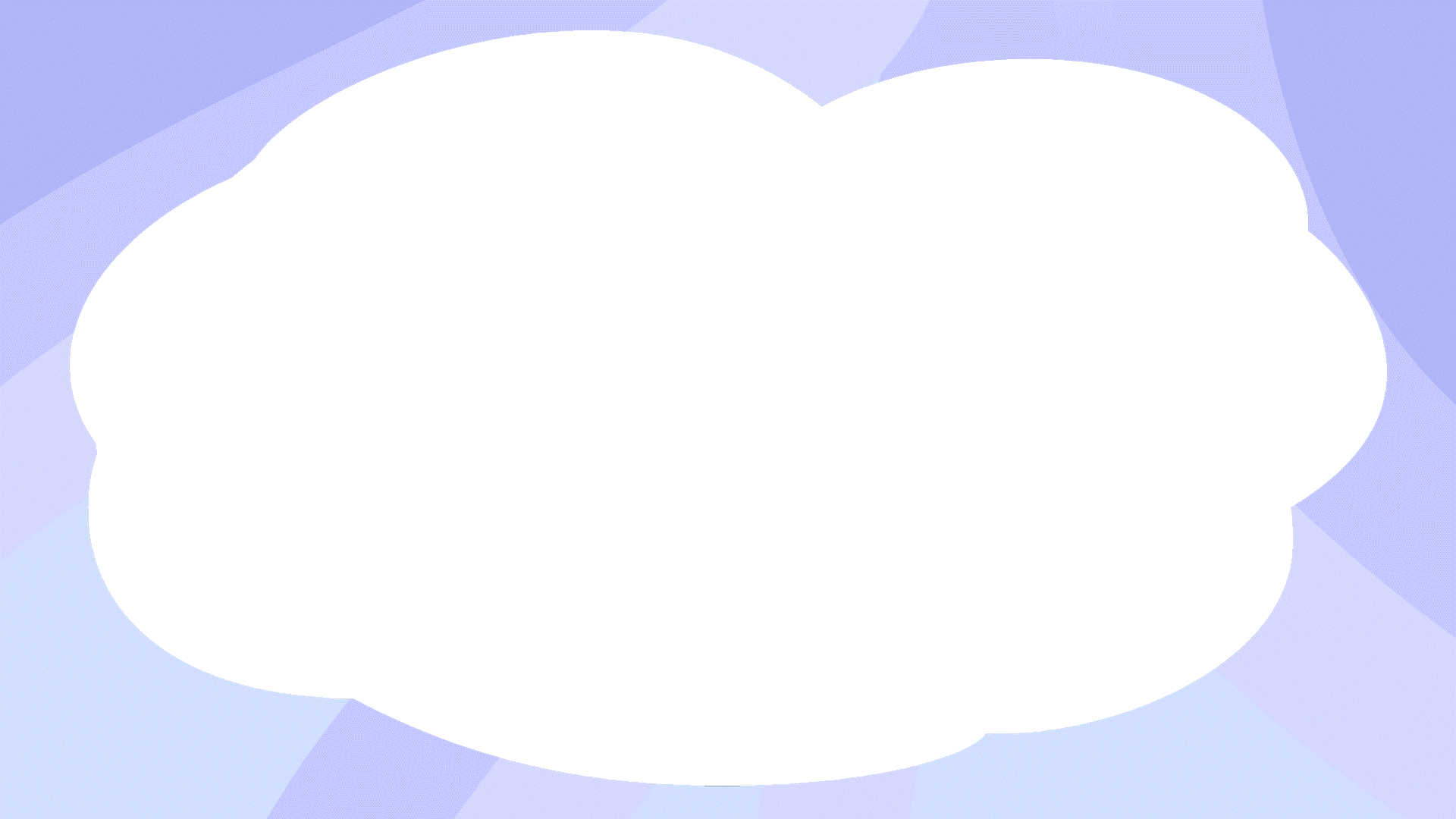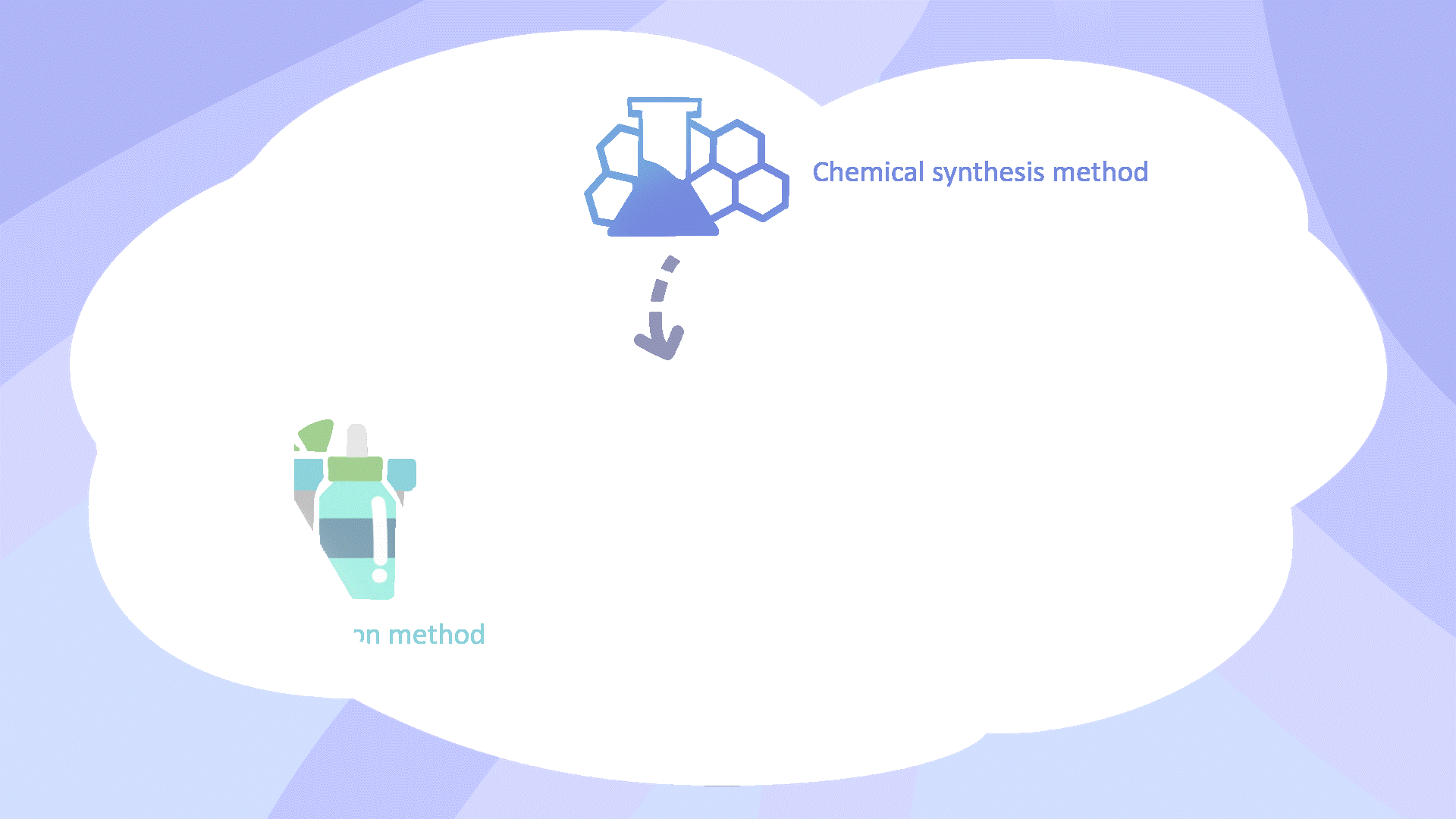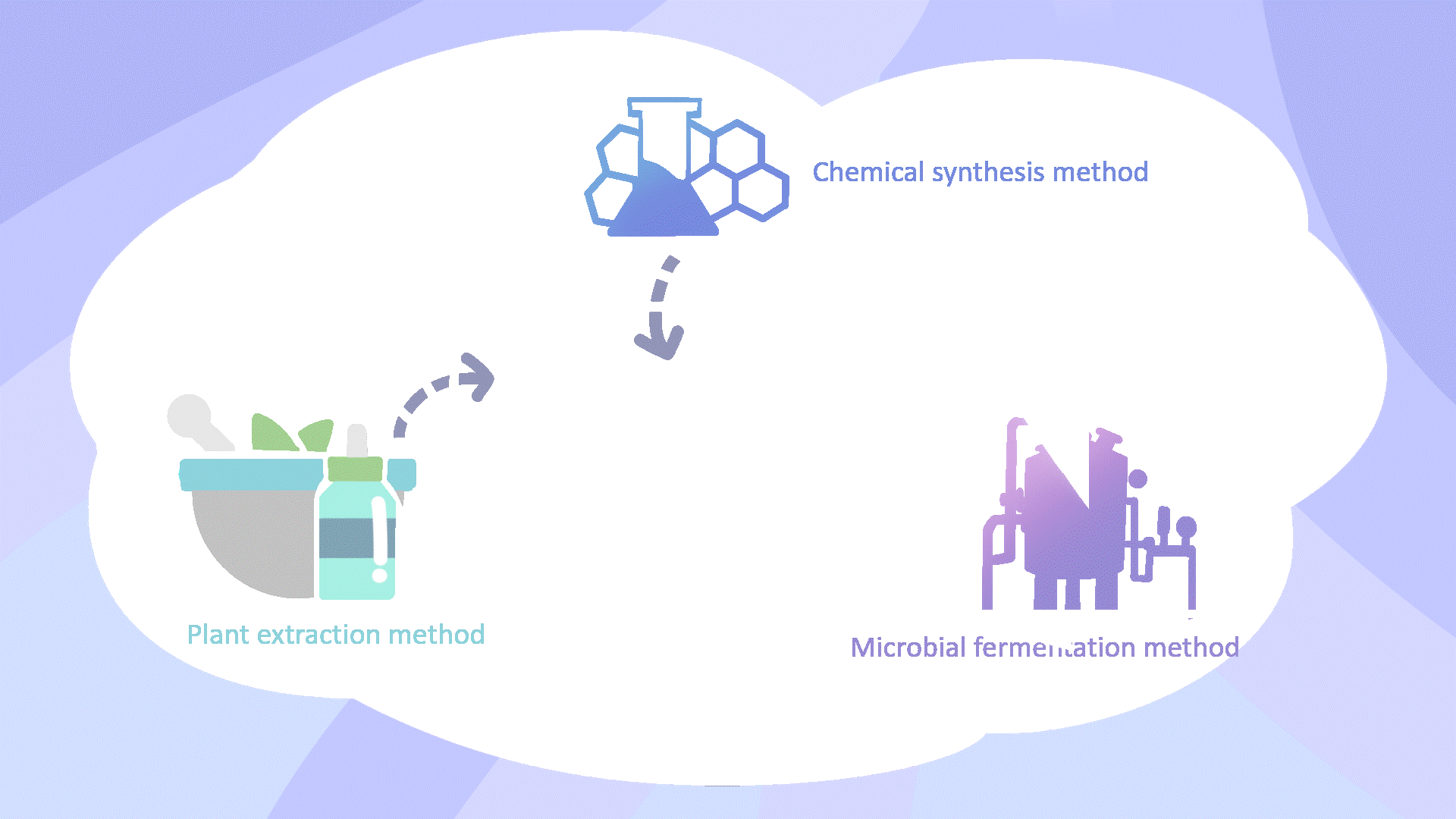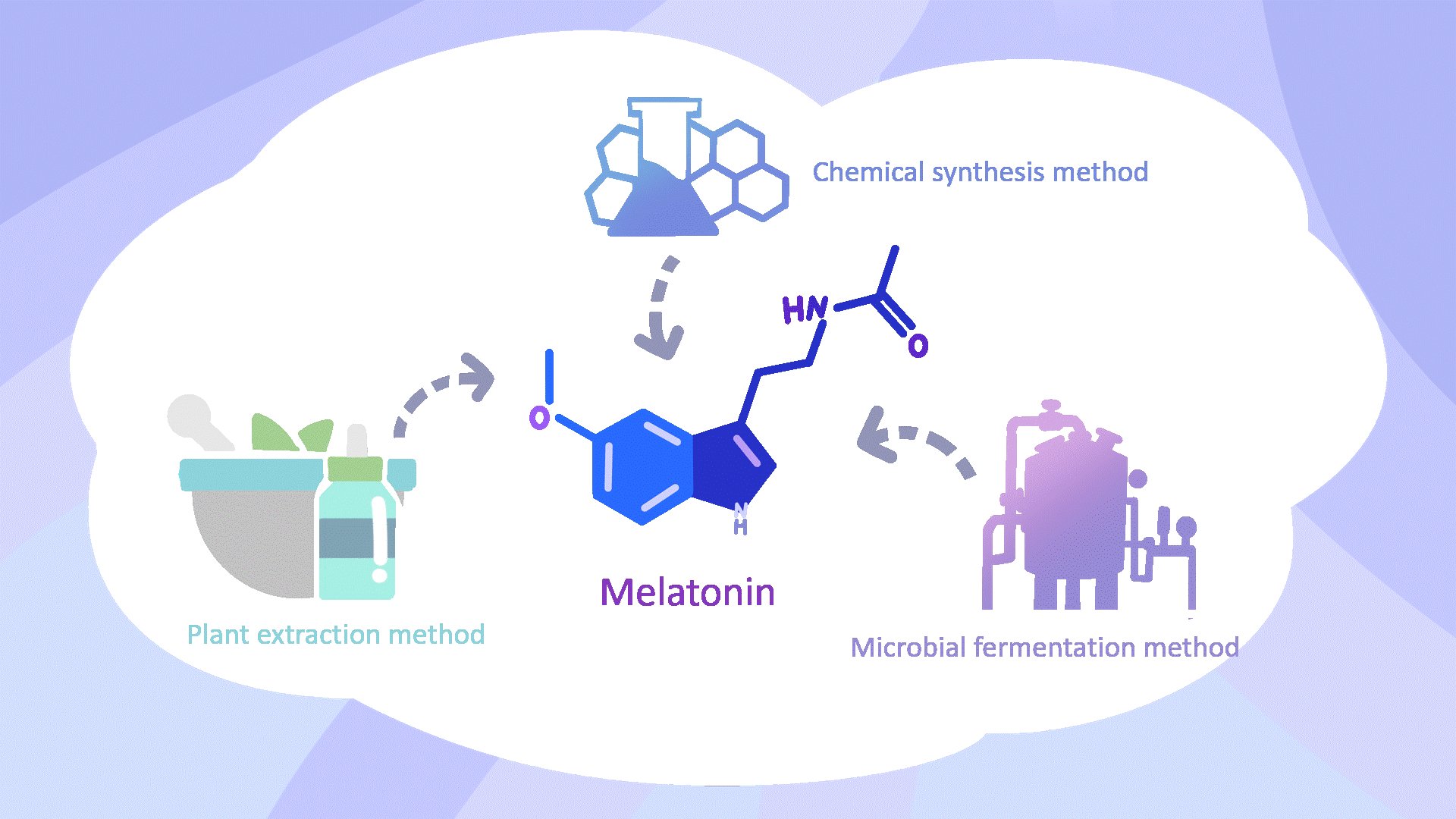
The Limitation of Biosynthetic Melatonin
Dual Constraints: Precursor Supply and Enzyme Catalysis
- Insufficient precursor supply: L-tryptophan, the direct precursor of MT, is produced via the shikimate pathway. Its limited metabolic flux restricts MT synthesis8,9.
- Low catalytic efficiency: Key enzymes such as tryptophan hydroxylase (L-Trp → 5-hydroxytryptophan) and arylalkylamine N-acetyltransferase (serotonin → N-acetylserotonin) have weak activity, reducing overall synthesis efficiency8,9.
Bottleneck in the Final Methylation Step
The final methylation step, catalyzed by Arabidopsis thaliana caffeic acid O-methyltransferase (AtCOMT), converts N-acetylserotonin into MT. This is the rate-limiting reaction in the pathway. Although previous AtCOMT mutants exhibited 9.5-fold higher activity than wild-type, they still fail to meet industrial demands for efficient and stable catalysis. Limited enzyme activity can cause intermediate accumulation, reducing MT yield and purity10.

Our Research
This study enhanced MT production by improving both L-tryptophan availability and AtCOMT catalytic efficiency. L-tryptophan production reached 3.99 g/L through upstream gene knockouts, overexpression, whole-genome mutagenesis, and tnaC-biosensor screening, achieving a 1609.9-fold increase over the wild-type BW25113. A growth-coupled high-throughput screening system based on the synthetic methyl cycle was used to select high-activity AtCOMT mutants. The best variant showed 84% higher catalytic efficiency than the highest literature-reported value, demonstrating the effectiveness of combining directed evolution, biosensors, and computational design for MT biosynthesis.

References
- Wang, L., Deng, Y., Gao, J. et al. Biosynthesis of melatonin from l-tryptophan by an engineered microbial cell factory. Biotechnol Biofuels 17, 27 (2024). DOI: 10.1186/s13068-024-02476-7
- Sun, C., Liu, L., Wang, L., Li, B., Jin, C., and Lin, X. Melatonin: A master regulator of plant development and stress responses. J Integr Plant Biol. 63, 126–145 (2021). DOI: 10.1111/jipb.12993
- Inoue, K., Takano, H., Shimada, A., & Satoh, M. Metallothionein as an anti-inflammatory mediator. Mediators of Inflammation, 2009, 101659. DOI: 10.1155/2009/101659
- Megha, K. B., et al. Significance of Melatonin in the Regulation of Circadian Rhythms and Disease Management. Mol Neurobiol. 61, 5541–5571 (2024). DOI: 10.1007/s12035-024-03915-0
- Xie, X., et al. Melatonin biosynthesis pathways in nature and its production in microorganisms. Synth Syst Biotechnol. 7, 544–553 (2022). DOI: 10.1016/j.synbio.2021.12.011
- He, L., Li, J. L., Zhang, J. J., Su, P., & Zheng, S. L. Microwave Assisted Synthesis of Melatonin. Synth Commun. 33, 741–747 (2003). DOI: 10.1081/SCC-120016317
- Meng, X., Li, Y., Li, S. et al. Dietary Sources and Bioactivities of Melatonin. Nutrients 9, 367 (2017). DOI: 10.3390/nu9040367
- Luo, H., Schneider, K., Christensen, U., Lei, Y., Herrgard, M., and Palsson, B. Ø. Microbial Synthesis of Human-Hormone Melatonin at Gram Scales. ACS Synth Biol. 9, 1240–1245 (2020). DOI: 10.1021/acssynbio.0c00065
- Zimmermann, P., Kurth, S., Pugin, B. et al. Microbial melatonin metabolism in the human intestine as a therapeutic target for dysbiosis and rhythm disorders. Npj Biofilms Microbiomes 10, 139 (2024). DOI: 10.1038/s41522-024-00605-6
- Wang, W., Su, S., Wang, S., Ye, L., and Yu, H. Significantly improved catalytic efficiency of caffeic acid O-methyltransferase towards N-acetylserotonin by strengthening its interactions with the unnatural substrate’s terminal structure. Enzyme Microb Technol. 125, 1–5 (2019). DOI: 10.1016/j.enzmictec.2019.02.005
