Method wet lab
Protein Expression
Strains inoculation
1. Turn on the ultra-clean table UV lamp for 30 min in advance, and set the shaker speed and temperature at 220rpm and 37 ℃.
2. Open the sterilized culture medium, add 50 μL of kanamycin stock solution (30 mg/mL) to the conical flask, and shake for a thorough mix.
3. Take 7 mL of LB culture medium to the test tube.
4. Add about 30 μL of -80 ℃ lyophilized monoclonal strain into the test tube with 7 mL of culture medium.
5. Put the test tube inside the shaker and set the parameter at 37 ℃, 220 rpm for an overnight incubation, which is about 12 hr.
6. Store the culture medium at 4 ℃.
100 mL LB medium formulation
2. Open the sterilized culture medium, add 50 μL of kanamycin stock solution (30 mg/mL) to the conical flask, and shake for a thorough mix.
3. Take 7 mL of LB culture medium to the test tube.
4. Add about 30 μL of -80 ℃ lyophilized monoclonal strain into the test tube with 7 mL of culture medium.
5. Put the test tube inside the shaker and set the parameter at 37 ℃, 220 rpm for an overnight incubation, which is about 12 hr.
6. Store the culture medium at 4 ℃.
100 mL LB medium formulation

Expanded culture
1. Remove the test tube from the shaker and place it on the ultra-clean table.
2. Take out the test tube containing the bacterial fluid and transfer 1-2 mL of it into a conical flask containing the sterilized TB culture medium.
3. Repeat the procedures with the other test tube and the conical flask.
4. Place the two conical flasks in the shaker and set the parameter at 37 ℃, 220 rpm incubation, about 3-4 h,OD=0.6.
5. Preserve the strain.
900 mL TB medium formulation
2. Take out the test tube containing the bacterial fluid and transfer 1-2 mL of it into a conical flask containing the sterilized TB culture medium.
3. Repeat the procedures with the other test tube and the conical flask.
4. Place the two conical flasks in the shaker and set the parameter at 37 ℃, 220 rpm incubation, about 3-4 h,OD=0.6.
5. Preserve the strain.
900 mL TB medium formulation

IPTG induction
1. Take out the conical flasks from the shaker and put them into the ultra-clean table together with IPTG.
2. Add 0.5nM IPTG under the surface of the culture medium and repeat the procedure.
3. Place the conical flasks back into the shaker and incubate at 16 ℃, 220 rpm for 16 h.
2. Add 0.5nM IPTG under the surface of the culture medium and repeat the procedure.
3. Place the conical flasks back into the shaker and incubate at 16 ℃, 220 rpm for 16 h.
Collection
1. Centrifuge the bacterial fluid and discard the supernatant.
2. Add 5mL of 20mM imidazole respectively and mix well with blowing. Then transfer them to the same tube.
3. Add 200ul PMSF.
4. Preserve it at -80°C.
2. Add 5mL of 20mM imidazole respectively and mix well with blowing. Then transfer them to the same tube.
3. Add 200ul PMSF.
4. Preserve it at -80°C.
Purification
Ultrasonication
1. Take out the bacteria and melt it at room temperature.
2. Place the tube that contains the bacterial fluid in a beaker and bathe it with ice water. Then sonicate the tube until the bacterial fluid is clear.
2. Place the tube that contains the bacterial fluid in a beaker and bathe it with ice water. Then sonicate the tube until the bacterial fluid is clear.
Centrifugation
3. Centrifuge the bacterial fluid (10000×g, 30min).
Pass through Ni column
1. Equilibrate the Ni column with distilled water and then binding buffer.
2. Elute the protein with 20mM, 30mM, 60mM, 150mM, and 300mM imidazole respectively, and collect it at the end.
3. Ascertain the result of protein purification by SDS-PAGE electrophoresis.
8% SDS-PAGE Glue Formulation
2. Elute the protein with 20mM, 30mM, 60mM, 150mM, and 300mM imidazole respectively, and collect it at the end.
3. Ascertain the result of protein purification by SDS-PAGE electrophoresis.
8% SDS-PAGE Glue Formulation
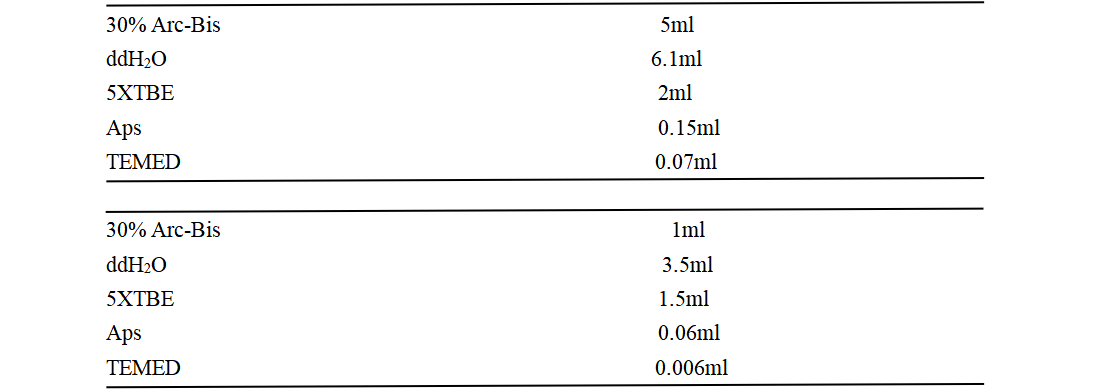
SDS-PAGE sample system configuration

Ultrafiltration
1. Using a 10 KDa ultrafiltration tube, add the collection solution (placed on an ice box) (Max=400 μL) and centrifuge at high speed (4 °C, 12000 × g, 4 min).
2. Discard the effluent, add the collection solution to the ultrafiltration tube, and repeat these steps until the entire collection solution is ultrafiltered.
3. Add protein diluent into the ultrafiltration tubes, blow and mix, and ultrafiltrate it. Repeat three times.
4. Bradford method for concentration measurement.
5. Sub-pack the solution and store at -80°C.


2. Discard the effluent, add the collection solution to the ultrafiltration tube, and repeat these steps until the entire collection solution is ultrafiltered.
3. Add protein diluent into the ultrafiltration tubes, blow and mix, and ultrafiltrate it. Repeat three times.
4. Bradford method for concentration measurement.
5. Sub-pack the solution and store at -80°C.
Enzyme activity verification
PAGE Verification
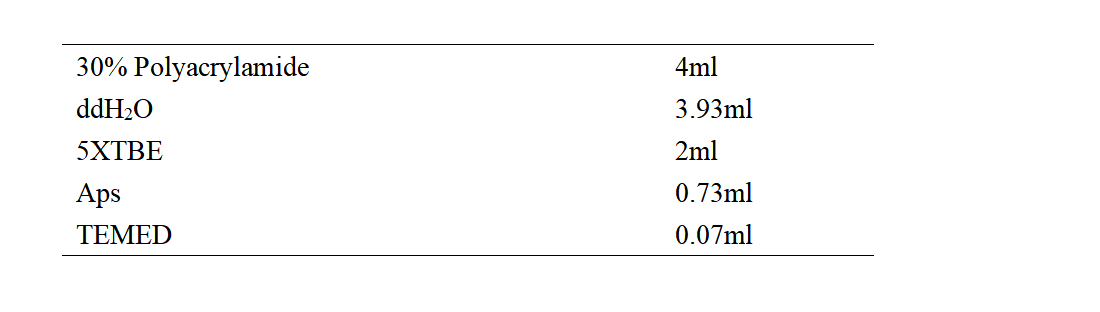
1. Configure PAGE.
PAGE Glue Formulation
PAGE Glue Formulation

2.Configuration 50 μ l reaction system. Reaction at 37 ℃ for 30min, centrifugation at 12000 rpm for 5min, and take the supernatant.

3. PAGE sample system configuration

FQ reporter cleavage by different Cas-crRNA in the presence of DNA targets
1. Set up the following 20 μl incubation system:
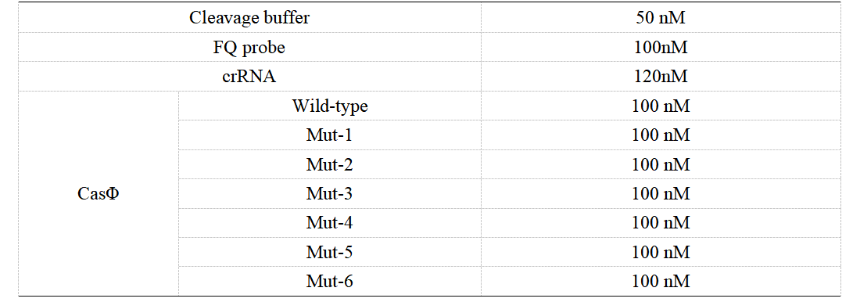
Dissolved in ddH2O, the mixture was incubated at 37℃ for 30min.
2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul:

3. Evenly mix the system and add it to 96-hole plates, 20 μl for each hole. Fluorescence signals were obtained every 2 minutes at 37°C.
FQ reporter cleavage by Cas-crRNA in the presence of DNA targets with different concentrations
1. Set up the following 20 μl incubation system:
Dissolved in ddH2O, the mixture was incubated at 37℃ for 30min.
 2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul:
2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul:
Dissolved in ddH2O, the mixture was incubated at 37℃ for 30min.
 2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul:
2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul: 
3. Evenly mix the system, add it to 96-hole plates, 20 μl for each hole. Fluorescence signals were obtained every 2 minutes at 37°C.
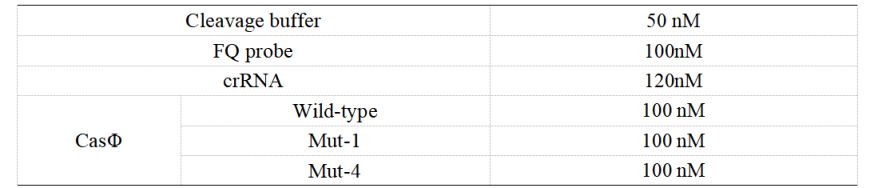
FQ reporter cleavage by Cas-crRNA in the presence of DNA targets with single-base mismatch.
1. Set up the following 20 μl incubation system:

Dissolved in ddH2O, the mixture was incubated at 37℃ for 30min.
2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul:
3. Evenly mix the system, add it to 96-hole plates, 20 μ l for each hole. Fluorescence signals were obtained every 2 minutes at 37°C.
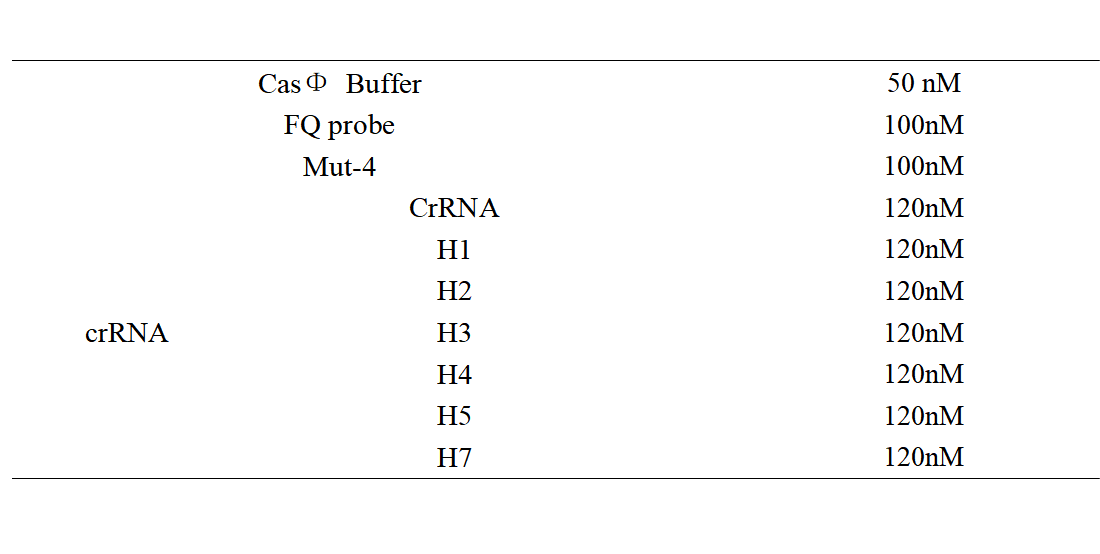
FQ reporter cleavage by Mut-4 with hairpin structure crRNA.
1. Set up the following 20 μl incubation system:
Dissolved in ddH2O, the mixture was incubated at 37℃ for 30min.
2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul:

3. Evenly mix the system, add it to 96-hole plates, 20 μ l for each hole. Fluorescence signals were obtained every 2 minutes at 37°C.
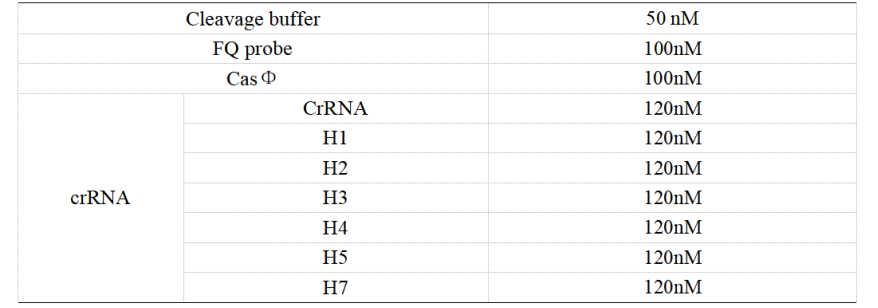
FQ reporter cleavage with samples containing 50% to 0% DNA mutations.
1. Set up the following 20 μl incubation system. The normal crRNA is added to WT, while the H4 is added to Mut-4.
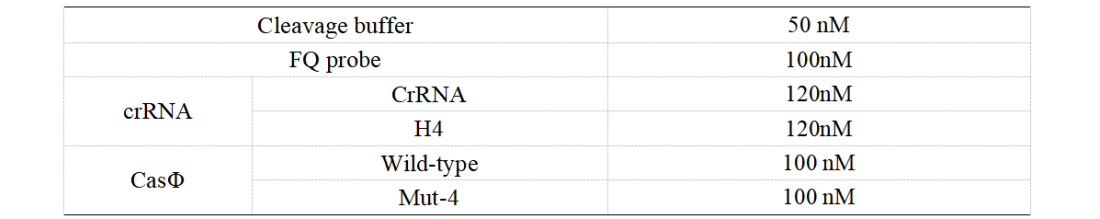
Dissolved in ddH2O, the mixture was incubated at 37℃ for 30min.
2. Add following reagents to each protein reaction system after incubation to the certain concentration, and the volume of the overall system is 60ul:

3. Evenly mix the system, add it to 96-hole plates, 20 μ l for each hole. Fluorescence signals were obtained every 2 minutes at 37°C.