Basic Parts
-
1. Part:BBa_K5161001
DnaK+Flagx3
Dnak is a molecular chaperone protein composed of 250 amino acids. We add it to the sequence with the aim of forming a recombinant protein with GLP-1. The binding and disaggregation of Dnak with ClpB is a dynamic process that varies with the ATP concentration within the bacteria. When ATP concentration rises (i.e., when elevated blood sugar leads to increased bacterial metabolism), Dnak disaggregates with ClpB, allowing the release of the GLP-1 recombinant protein into the extracellular space, thus achieving blood sugar concentration-dependent regulatory function. Flagx3 is a tagged sequence used for the screening and identification of fusion proteins in Western blot.
Description
In E.coli, DnaK and ClpB are responsible for dissolving protein aggregates, with DnaK binding directly to the aggregate and recruiting the disaggregase ClpB . In the absence of DnaK, ClpB has been shown to have little disaggregation activity, but after directly binding with DnaK, the DnaK-ClpB complex shows high efficiency of disaggregation.
Usage and Biology
We fused DnaK, a key protein that leads to bacterial persistent resistance, with GLP-1. In the hyperglycemic state, glucose enters the bacteria and is used for aerobic respiration, producing more ATP, which reduces the affinity of ClpB-Dnak and depolymerizes the GLP-1 recombinant protein and releases it outside the cell. In the hypoglycemic state, the ratio of ATP to ADP decreases, the GLP-1 recombinant protein repolymerizes, and extracellular secretion is stopped.
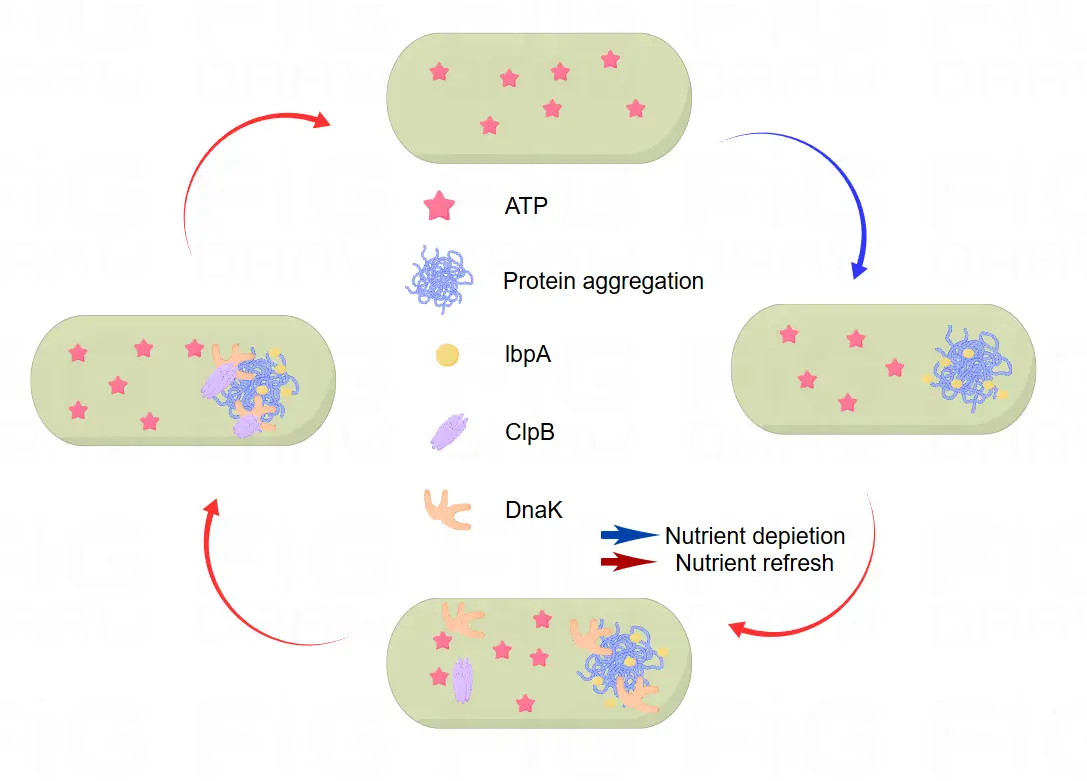
Dynamic changes in ATP concentrations within bacteria promote the formation and depolymerization of protein precipitate aggregates Characterization
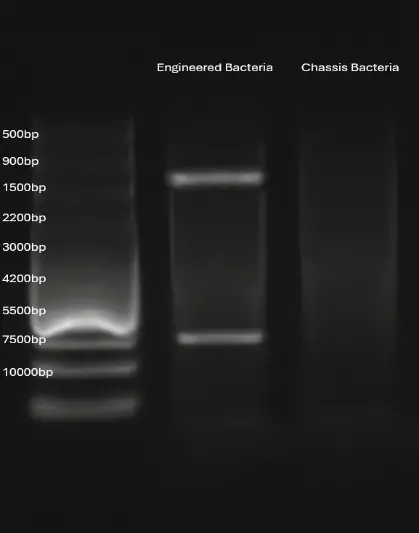
Agarose gel electrophoresis: Dnak fragments digested using enzymes The results of the agarose gel electrophoresis show that the plasmid in the engineered bacteria, after digestion with the restriction endonucleases BspDI and PuvI, produced two fragments of 1400 bp and 7500 bp, which are consistent with the expected results (as the restriction enzyme sites are located on the Dnak fragment). This indicates that Dnak was successfully ligated into the plasmid and introduced into the bacteria.
Reference
Pu Y, Li Y, Jin X, Tian T, Ma Q, Zhao Z, Lin S, Chen Z, Li B, Leake MC, Lo CJ, Bai F, ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance, Molecular Cell73 (2019), pp. 1-14 (November. 21.2018 online)
-
2. Part:BBa_K5161002
Flexible long Linker (Gly-Gly-Gly-Ser)x3
With the development of molecular biology and synthetic biology, recombinant technology and fusion protein technology have become more and more mature. In order to achieve multifunctional protein multi-targeting, or to combine multiple proteins to coagulate into fusion proteins, the role of flexible protein peptides becomes more and more important. The sequence produced the aminoacids
Gly-Gly-Gly-Ser-Gly-Gly-Gly-Ser-Gly-Gly-Gly-Ser.Usage and Biology
This is a connector that connects the proteins, and it has no effect on the parts to which it is attached. The sequence of the linker is designed for amino acids that do not interact with other amino acid residues. The sequence of the linker is designed for amino acids that do not interact with other amino acid residues. The amino acids glycine and serine are zwitterionic and hydrophilic, and these properties make them good candidates for linker repeats.The length of the connector is important to guarantee the independent function of the two connected parts. When the connector is too short, there may be spatial interference between the parts, and when the connector is too long, it can lead to structural instability. In addition, it is important that Linker has some flexibility.
Composite Part
Part:BBa_K5161003
GLP-1 Recombinant Protein
The recombinant protein includes the GLP-1 fragment, pelB, Dnak, a flexible linker peptide sequence, and a Flag tag sequence.It is also an improved part of the 2024 Squirrel-CHN iDEC team Pel-GLP-1 Coding.
Usage and Biology
In this particular project, the overarching purpose of designing the GLP-1 recombinant protein is to enhance the adaptability and flexibility of Pel-GLP-1 Coding with the aim of achieving a glucose concentration-responsive functional release of GLP-1. Specifically, the release switch for GLP-1 is activated when the blood glucose levels ascend, which then proceeds to lower the glucose levels. Conversely, it is turned off when the glucose levels are low. The recombinant protein is composed of several key components, including the GLP-1 fragment, pelB, Dnak, a flexible linker peptide sequence, and a Flag tag sequence. As previously mentioned, pelB functions as a signal sequence that directs GLP-1 for extracellular secretion. Dnak, on the other hand, is the crucial core component of the glucose regulatory switch.
The mechanism of its action is as follows: When the glucose concentrations increase, the elevated internal glucose accelerates the metabolic process, which in turn elevates the ATP/ADP ratio. This leads to a weakening of the chaperone interaction between Dnak and ClpB. As the binding affinity reduces, the recombinant protein disassembles. Subsequently, this disassembly guides the release of the recombinant protein into the extracellular space. This intricate process demonstrates the sophisticated design and functionality of the GLP-1 recombinant protein system, which holds great potential for applications in the field of glucose regulation and related biomedical research. It provides a novel approach to precisely control the release of GLP-1 in response to changes in glucose levels, potentially leading to the development of more effective therapeutic strategies for conditions related to glucose metabolism disorders. Further investigations and optimizations of this system could pave the way for significant advancements in the treatment and management of such disorders, contributing to improved healthcare outcomes in the future.
Modulation and Holisticness
As previously mentioned, the components of the recombinant protein are derived from diverse organisms. Each part is meticulously selected to fulfill a specific role, with the aim of ensuring the overall functionality and responsiveness of the protein in regulating blood glucose levels. The GLP-1 fragment, for instance, is crucial for its direct interaction with the relevant physiological processes involved in glucose metabolism. PelB, sourced from a particular organism, is chosen as it effectively functions as a signal sequence to guide the GLP-1 towards extracellular secretion, facilitating its release into the appropriate environment to exert its regulatory effects. Dnak, originating from another organism, plays a central role in the glucose regulatory switch. Its specific properties and interactions within the protein complex are essential for sensing changes in glucose concentrations and initiating the corresponding responses. The flexible linker peptide sequence provides the necessary flexibility and conformational stability, allowing the different components to interact and function in a coordinated manner. The Flag tag sequence, although seemingly small in comparison, also has its significance, perhaps in terms of facilitating detection and identification of the recombinant protein during research and experimentation. Together, these components from different organisms work in harmony to endow the recombinant protein with the ability to precisely respond to changes in glucose levels and carry out its regulatory function, which holds great promise for applications in the field of diabetes treatment and glucose homeostasis research.
Careful Design
In the meticulous design of the recombinant sequence, we gave thorough consideration to the positioning of each segment with the intention of optimizing the glucose regulation functionality to the greatest extent. PelB is strategically placed at the N-terminus, as this positioning is crucial for facilitating the efficient secretion of GLP-1. Right after PelB comes the GLP-1 sequence, which acts as the central and essential component for carrying out the primary function of glucose regulation. Following the GLP-1 sequence, we deliberately included a flexible linker peptide sequence, specifically (GGGSGGGSGGGS). This linker sequence is designed to ensure a seamless and smooth transition to the subsequent Dnak sequence, enabling proper interaction and functionality between the adjacent components. After Dnak, we added a 3xFlag tag, which serves a vital purpose in the identification of the recombinant protein expression. This tag allows for easy detection and tracking of the protein during various experimental procedures and analyses. This comprehensive approach to sequence design and verification is essential to guarantee the successful development and application of the recombinant protein in the field of glucose regulation research and related biomedical applications.
Characterization
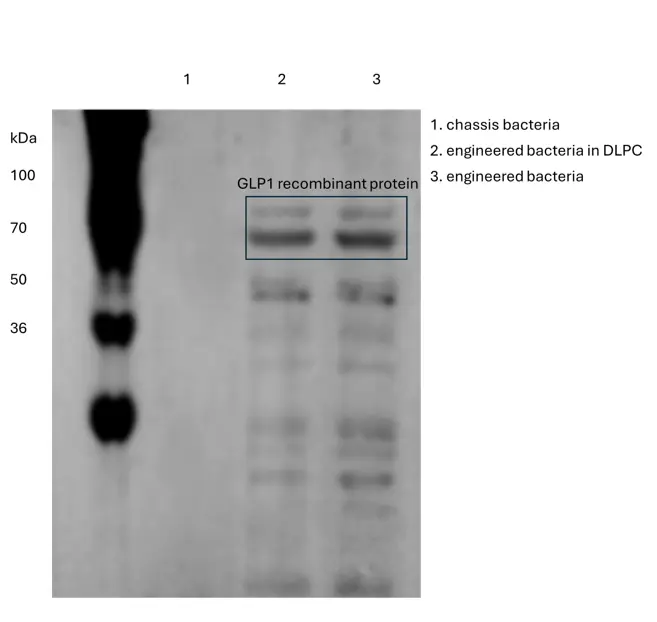
-
1. The Western blot results clearly demonstrate that the GLP-1 recombinant protein was successfully detected in both the unmodified and DLPC vesicle-encapsulated engineered bacterial supernatants. This finding is of significant importance as it provides evidence of the presence of the target protein in these different sample conditions. The measured molecular weight of the GLP-1 recombinant protein is determined to be in the range of 74 - 76 kDa. This precise molecular weight characterization is crucial for further analysis and understanding of the protein's properties and functions. It allows for comparison with the expected molecular weight based on its amino acid sequence and any post-translational modifications that may occur. Additionally, the knowledge of the protein's molecular weight in this context can assist in identifying potential interactions or conformational changes that might be associated with its encapsulation in the DLPC vesicles compared to its unmodified state in the bacterial supernatants. Overall, these results lay a solid foundation for further investigations into the biological activity, stability, and potential applications of the GLP-1 recombinant protein in various biomedical and research settings.
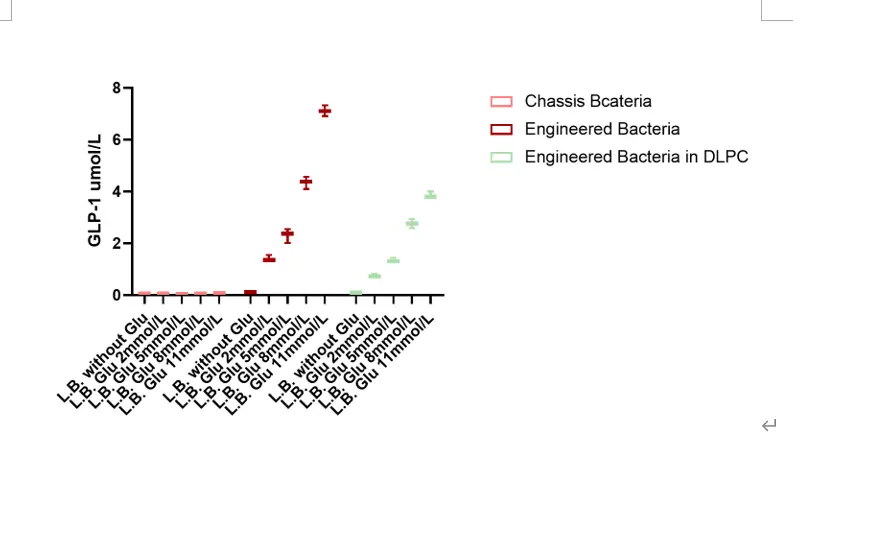
- 2. The results of the glucose gradient cultivation clearly indicate that, within the same time scale of 12 hours, the concentration of the GLP-1 recombinant protein in the supernatant of the engineered bacteria shows a notable increase as the initial glucose concentration in the medium rises. The secretion level of the GLP-1 recombinant protein from the unmodified engineered bacteria is remarkably higher compared to that from the DLPC-encapsulated engineered bacteria. This finding strongly demonstrates that the GLP-1 recombinant protein possesses a distinct functionality of release that is dependent on the glucose concentration. It implies that the presence and amount of glucose in the environment can significantly influence the release behavior of the GLP-1 recombinant protein, which is an important characteristic that may have implications for various applications in the fields of biotechnology and medicine. For example, this glucose concentration-dependent release functionality could potentially be utilized in the development of new drug delivery systems or in the study of metabolic pathways related to glucose regulation. Further research could explore the underlying mechanisms of this dependency and how it can be optimized to achieve more precise and effective control over the release of the GLP-1 recombinant protein. Overall, these results open up new avenues for investigating the interaction between glucose and the engineered bacteria producing the GLP-1 recombinant protein and hold great promise for future advancements in related scientific and medical research.