Acylation is an important and widespread strategy for the functional control of biomolecules. Malonylation and succinylation, harboring a terminal carboxyl group, have been demonstrated to be important post-translational modifications of proteins. Notably, for small-molecule natural products, malonylation/succinylation could alter their chemical stability and biological activity. For example, the 3-O-succinyl-28-O-benzyl oleanolate derivative presents greater cytotoxicity and apoptosis effects than its natural precursor, oleanolic acid, or its benzyl derivative. 7-O-succinyl macrolactin A (SMA) and 7-O-malonyl macrolactin A (MMA) have superior antibacterial activities compared to that of macrolactin A (MLN A). However, few microbial natural products are modified by malonyl or succinyl groups.
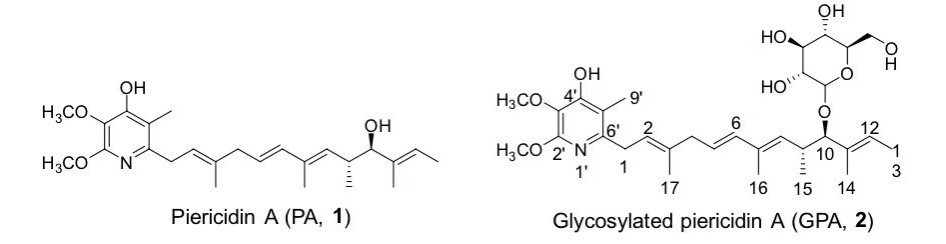
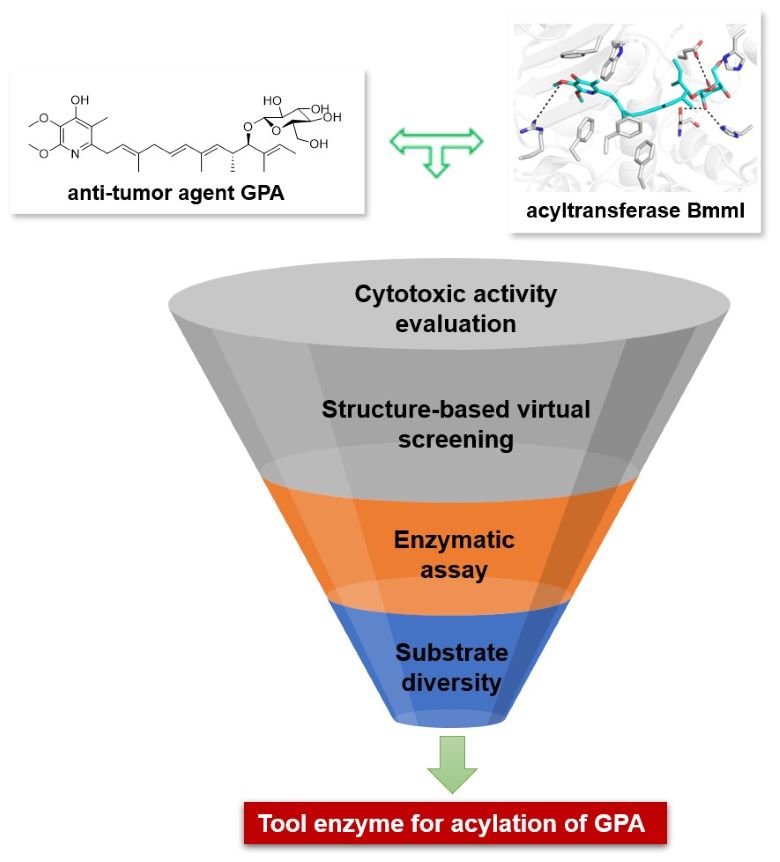
Piericidins belong to a family of a-pyridone antibiotics, which feature a highly substituted pyridone core with a lipophilic side chain (Fig 1). Because of their structural resemblance to coenzyme Q10, piericidins have been demonstrated to be potent NADH-ubiquinone oxidoreductase (complex I) inhibitors in the mitochondrial electron transport chain. Among them, 10-glucopiericidin A1 (GPA, 2) is a lead compound with anti-renal cell carinoma efficacy (Fig 1). Considering that acyl groups often influence the bioactivities of natural products, in this article, we first evaluated the cytotoxic activity of malonylated GPA and found malonylation could improve the activity. Then the molecular docking studies were carried out to investigate the binding mode of GPA in BmmI. Furthermore, we used structure-based virtual screening to construct a saturated mutation library and selected suitable mutants for expression in E. coli Rossetta (DE3). Finally, mutants with enhanced malonylation activities were obtained and the catalytic activities of the mutant towards other acyl donors (methylmalonyl, succinyl) were assessed (Fig 2).