
The Bacillus-derived acyltransferase BmmI exhibited broad substrate flexibility, transferring malonyl group not only to the macrolide compounds, but also to the anti-tumor agent glycosylated piericidin A (GPA). In this project, we found that malonylation could improve the cytotoxic activity of GPA, indicating BmmI have a great potential to generate derivatives with higher activities. Therefore, we applied semi-rational directed evolution strategy to engineer BmmI for higher catalytic efficiency toward GPA. Our study provides foundation for further engineering of BmmI to obtain GPA derivatives with better bioactivities.
Read More
Cytotoxic activity evaluation of malonylated GPA
Read More
Identifying the candidate residues of Bmml for directed evolution
Read More
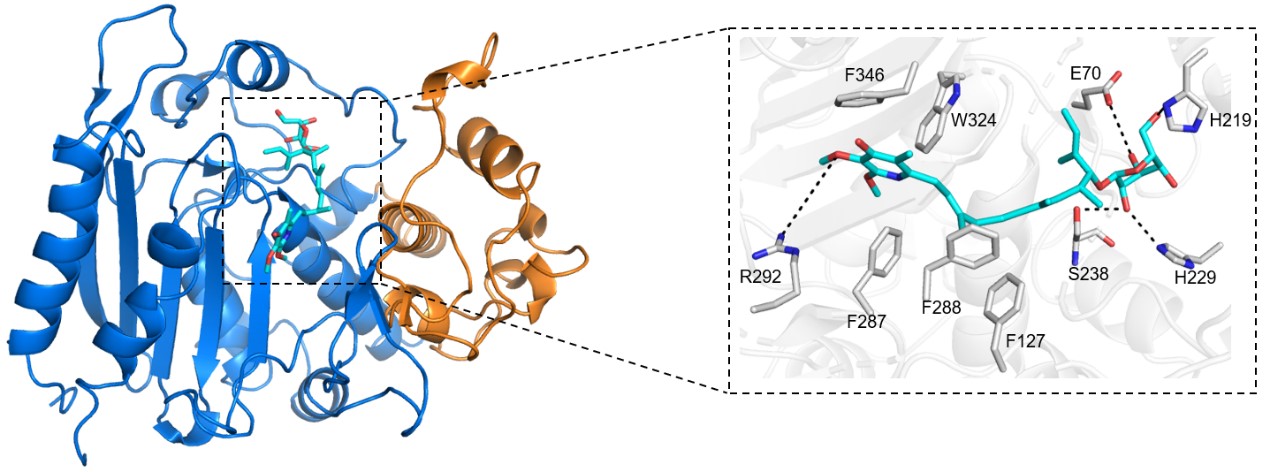
Structure-based virtual screening of Bmml
Read More
Construction and activity tests of mutants
Read More
In vitro characterization of the BmmI variant W324A with other acyl donors
Read More
Conclusion
Read More