



Paraxanthine (PX) is an important compound produced through the metabolism of caffeine. It is one of the metabolites of purine substances, and when we consume coffee, tea, or other caffeine-containing foods, approximately 80% of the caffeine in the body is metabolized into PX. This transformation primarily relies on an enzyme system in the liver called cytochrome P450 enzymes (CYP1A2), which are responsible for breaking down caffeine into PX1.

PX is not just a byproduct of caffeine metabolism; in many ways, it performs better than caffeine itself2.
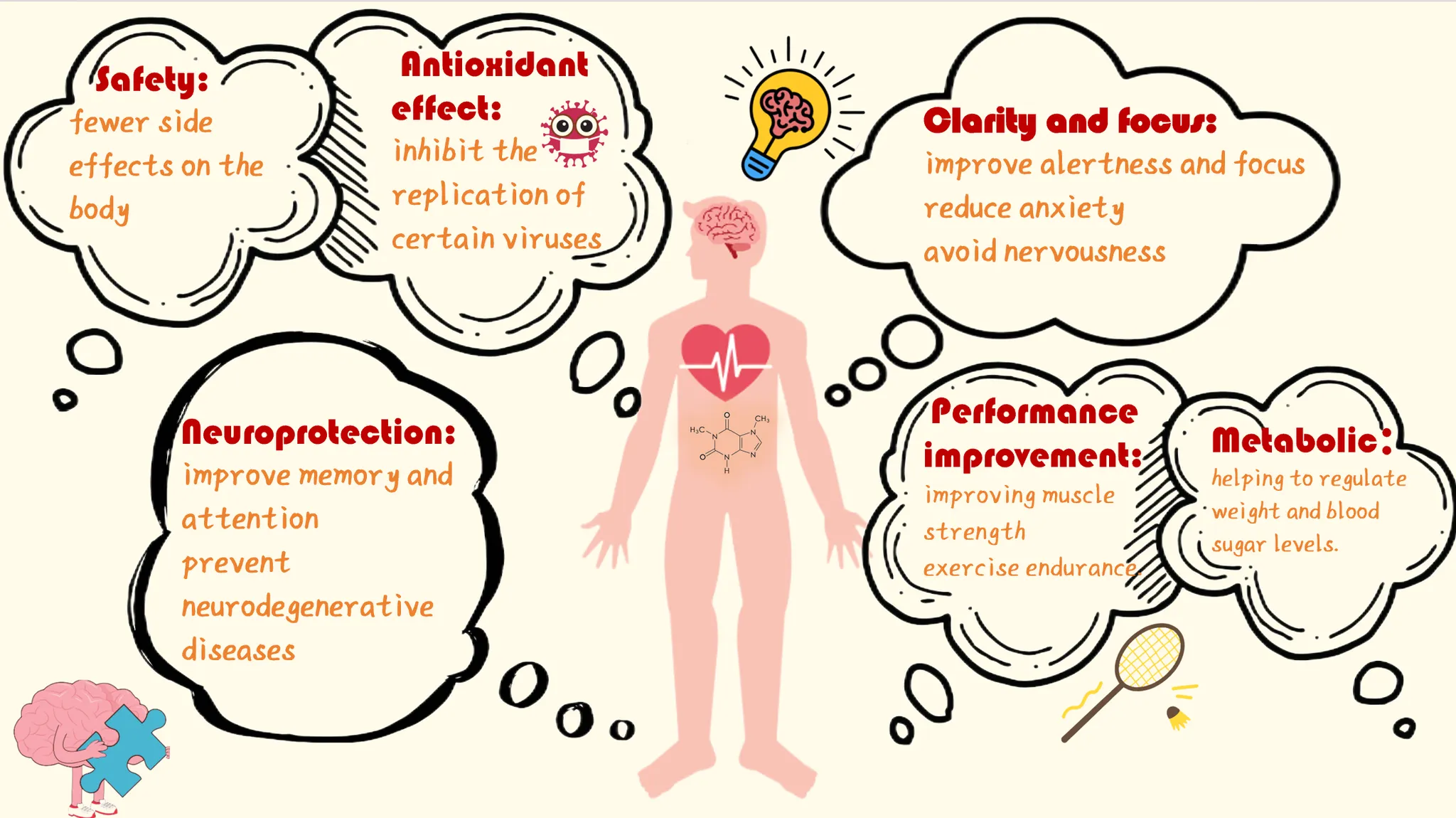
1.Alertness and Focus: Like caffeine, PX can increase wakefulness and concentration, but unlike caffeine, it reduces anxiety and avoids side effects such as palpitations or nervousness.
2.Neuroprotection: PX has neuroprotective properties, helping improve memory and attention and possibly preventing neurodegenerative diseases such as Alzheimer's and Parkinson's.
3.Improvement of Athletic Performance: PX has shown significant effects in the fitness domain by promoting the synthesis of important molecules like inosine and adenosine, which enhances muscle mass, strength, and endurance.
4.Metabolic Regulation: PX plays an essential role in purine metabolism, particularly in fast-growing cells like immune and cancer cells. It also influences fat and glucose metabolism, helping regulate body weight and blood sugar levels.
5.Safety: Compared to caffeine, PX has fewer side effects, particularly concerning DNA damage, genotoxicity, and cytotoxicity.
6.Antioxidant Effects: As an antioxidant, PX helps eliminate harmful substances in the body and may inhibit the replication of certain viruses, such as HIV-1.
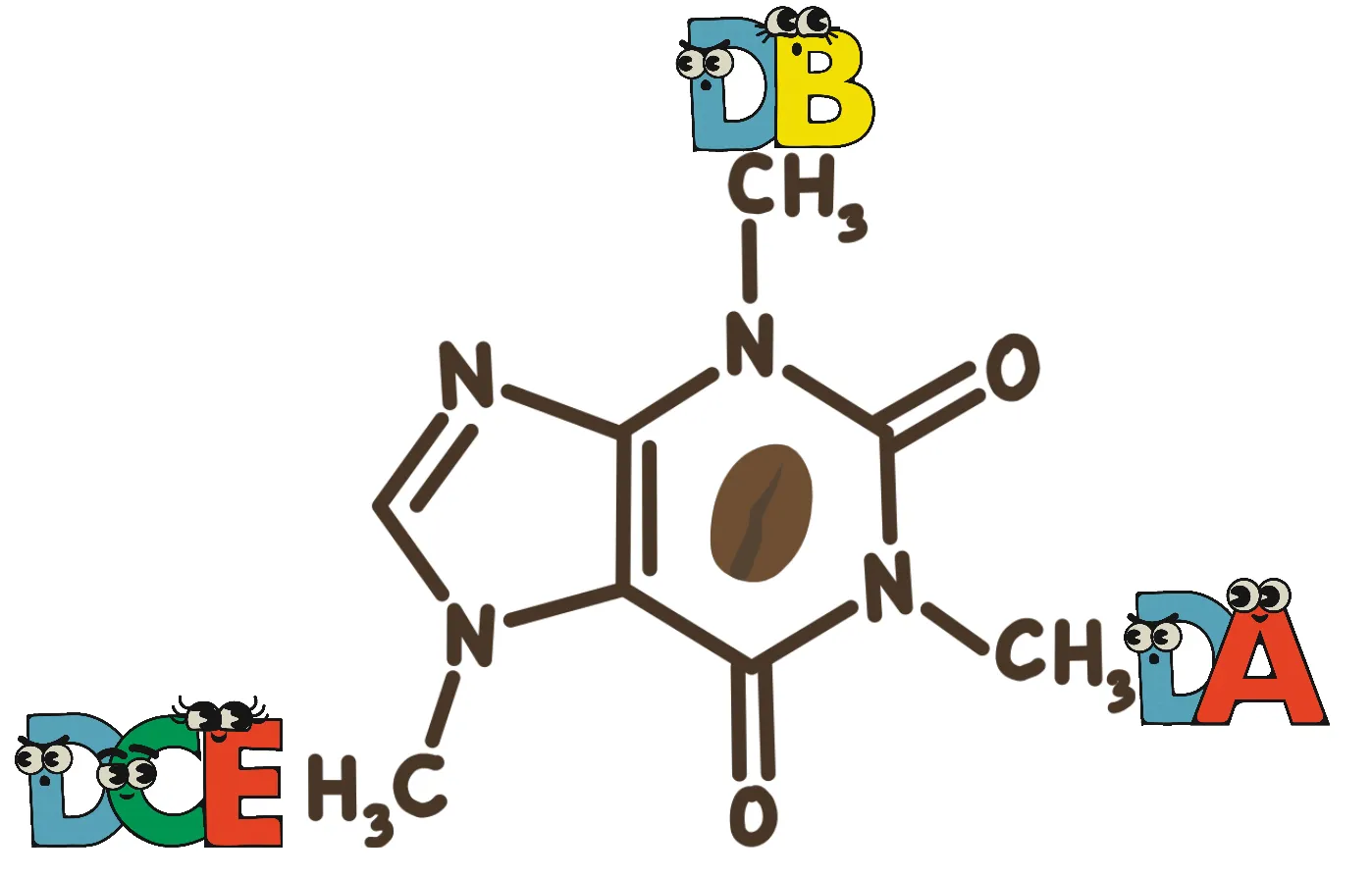
Despite its potential benefits, the high cost and low yield of PX are the primary challenges. PX is incredibly expensive, priced at 1,522 $/g, mainly due to its rarity in nature. In its natural state, PX is almost non-existent, making large-scale production through natural extraction nearly impossible. Currently, the primary method for PX production is chemical synthesis, particularly selective methylation reactions. However, this synthesis process is continuous, making it challenging to stop at a specific stage to obtain pure PX3.
Thus, biosynthesis methods with higher yields and environmental friendliness have gradually been applied in the production of PX. Research has found that a strain of Pseudomonas putida CBB5 (P. putida CBB5), isolated from soil, can utilize caffeine as its sole carbon and nitrogen source. This strain contains a gene cluster capable of degrading caffeine to xanthine through stepwise demethylation. The enzymes NdmA and NdmB catalyze the demethylation at the N1 and N3 positions, respectively, while the heterotrimeric reductase complex NdmCDE catalyzes the demethylation at the N7 position, ultimately degrading caffeine to xanthine4. Current biosynthesis methods for methylxanthines are largely based on this gene cluster, and efficient production of 1-MX, 3-MX, and 7-MX has already been achieved5. Since NdmB shares a high degree of homology with NdmA, researchers have engineered a double mutant of NdmA and replaced the loop structure near its active site with that of NdmB, thereby enabling N3 demethylation of caffeine to produce PX. However, the biosynthesis yield remains low, with a maximum of only 183.8 mg/L, accompanied by significant production of byproducts such as 7-MX. This low yield is mainly due to NdmB's poor efficiency in recognizing caffeine and its low substrate specificity, resulting in inefficient N3 demethylation6,7.
Overall, despite the promising potential applications of PX, achieving its large-scale use will require overcoming current production challenges and finding more efficient and cost-effective production methods.
In recent years, scientists have used a technique called “directed evolution” to improve enzyme function. This process accelerates natural selection in the lab, allowing researchers to repeatedly test and screen for proteins or enzymes with specific desirable functions. Compared to traditional methods, this approach is more flexible and efficient, enabling faster modifications to the biosynthesis process8. Additionally, we employed a semi-rational design approach. Semi-rational design is akin to making minor adjustments to key components of a complex machine, allowing us to quickly identify the optimal combination that improves its performance. This method is faster and more efficient than relying purely on random trials (irrational design) or complex calculations (rational design)9. Specifically, we also utilized a new screening tool—a whole-cell biosensor—that links enzyme activity to bacterial growth, making it easier to identify the best NdmB mutants10.
In this study, we used this technology to develop a screening system by observing bacterial growth to determine which enzyme mutations were more efficient at producing PX. We combined this with machine learning to analyze different enzyme mutation sites and identified a key site, Q289. We focused on modifying Q289 to assess its impact on PX production. Additionally, we optimized the gene's promoter to enhance enzyme efficiency, further increasing PX yield.
Through these methods, we successfully engineered the NdmB enzyme, making PX production more efficient. This research offers innovative strategies and tools for the efficient biosynthesis of PX and demonstrates the vast potential of directed evolution technology in biosynthesis.
1. Benowitz, N. L., Jacob, P., Mayan, H., & Denaro, C, Sympathomimetic effects of paraxanthine and caffeine in humans. Clinical Pharmacology & Therapeutics, 58(6), 684–691, (1995).
DOI: https://doi.org/10.1016/0009-9236(95)90025-x
2. Yoo, C., Xing, D., Gonzalez, D. E., Jenkins, V., Nottingham, K., Dickerson, B., Leonard, M., Ko, J., Lewis, M. H., Faries, M., Kephart, W., Purpura, M., Jäger, R., Wells, S. D., Liao, K., Sowinski, R., Rasmussen, C. J., & Kreider, R. B, Paraxanthine provides greater improvement in cognitive function than caffeine after performing a 10-km run. Journal of the International Society of Sports Nutrition, 21(1),(2024).
DOI: https://doi.org/10.1080/15502783.2024.2352779
3. Algharrawi, K. H. R., & Subramanian, M, Production of 7-methylxanthine from Theobromine by Metabolically Engineered E. coli. Iraqi Journal of Chemical and Petroleum Engineering, 21(3), 19–27,(2020).
DOI: https://doi.org/10.31699/ijcpe.2020.3.3
4. Algharrawi, K. H. R., Summers, R. M., Gopishetty, S., & Subramanian, M, Direct conversion of theophylline to 3-methylxanthine by metabolically engineered E. coli. Microbial Cell Factories, 14(1), (2015).
DOI: https://doi.org/10.1186/s12934-015-0395-1
5. Mock, M. B., Zhang, S., Pakulski, K., Hutchison, C., Kapperman, M., Dreischarf, T., & Summers, R. M, Production of 1-methylxanthine via the biodegradation of theophylline by an optimized Escherichia coli strain. Journal of Biotechnology, 379, 25–32, (2024).
DOI: https://doi.org/10.1016/j.jbiotec.2023.11.005
6. Mock, M. B., Mills, S. B., Cyrus, A., Campo, H., Dreischarf, T., Strock, S., & Summers, R. M, Biocatalytic production and purification of the high-value biochemical paraxanthine. Biotechnology and Bioprocess Engineering, 27(4), 640–651, (2022).
DOI: https://doi.org/10.1007/s12257-021-0301-0
7. Mills, S. B., Mock, M. B., & Summers, R. M. Rational Protein Engineering of Bacterial N-demethylases to Create Biocatalysts for the Production of Methylxanthines, (2021).
DOI: https://doi.org/10.1101/2021.12.17.472166
8. Chen, J., Wang, Y., Zheng, P., & Sun, J, Engineering synthetic auxotrophs for growth-coupled directed protein evolution. Trends in Biotechnology, 40(7), 773–776, (2022).
DOI: https://doi.org/10.1016/j.tibtech.2022.01.010
9. Romero, P. A., & Arnold, F. H., Exploring protein fitness landscapes by directed evolution. Nature Reviews. Molecular Cell Biology, 10(12), 866–876, (2009).
DOI: https://doi.org/10.1038/nrm2805
10. Williams, T. C., Pretorius, I. S., & Paulsen, I. T.,Synthetic Evolution of Metabolic Productivity Using Biosensors. Trends in Biotechnology, 34(5), 371–381,(2016).
DOI: https://doi.org/10.1016/j.tibtech.2016.02.002