Notebooks

On this page, all experiments performed in our wet lab are recorded by date. Detailed experiment protocols are provided on experiment page so not described on this page.
7.27
V.natriegens activation
XIRAN HU
Freeze-dried V.natriegens ATCC14048 ordered from CCIC was activated in LB+v2 salts overnight.
7.28
XIRAN HU
Glycerol stock
JINXUAN LU, JINPENG LI
Overnight culture of V.natriegens was stored as glycerol stock for later use.
Growth curve measurement
Three different mediums were inoculated with overnight V.natriegens culture to measure the growth curve. The result was as follows:
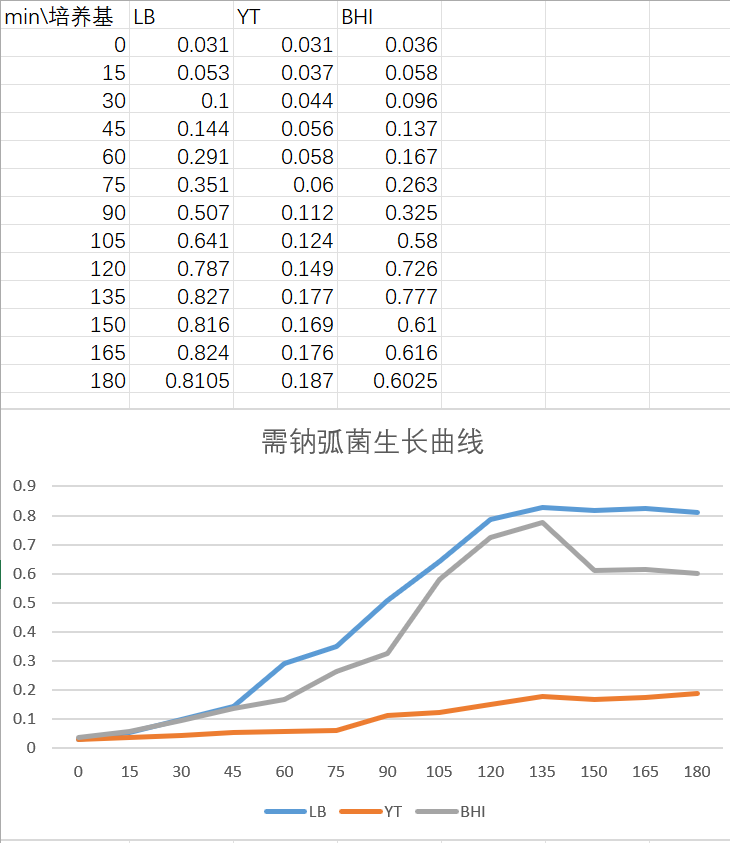
8.3
Stab culture activation
XIRAN HU
Top 10 strains carrying plasmids ordered from Azenta are activated from stab culture into liquid culture(LB broth with appropriate antibiotics).
8.4
ZIWEN XU
Plasmid extraction
XIRAN HU
Plasmids pGFP, pdCas9-sg1 and pPilin were extracted from Top 10 liquid culture. Concentration and quality of the plasmids were measured with Nanodrop.
pGFP |
pdCas9-sg1 |
pPilin |
|
|---|---|---|---|
| concentration(ng/μL) | 352.9 | 38.5 | 371.8 |
| OD260/280 | 1.89 | 1.77 | 1.90 |
| OD260/230 | 2.18 | 2.31 | 1.15 |
pdCas9-sg1 can not be used for transformation.
Glycerol stock
XIRAN HU
3 Top 10 strains were stored as glycerol stock for later use.
Chemical competent cell preparation
YUCHENG ZHANG
Prepare chemical competent V.natriegens for transformation.The chemical competent cells were stored at -80℃ until use.
8.5
Transformation of chemical competent cells
ZIWEN XU
The transformation was performed on home-made chemical competent V.natriegens and comercial DH5α with the following parameters
number |
Cell type |
plasmid name |
Plasmid concentration(μg/μL) |
Plasmid quantity(μL) |
|---|---|---|---|---|
| 1 | DH5α | pPilin | 371.8 | 1 |
| 2 | DH5α | pPilin | 37.18 | 2 |
| 3 | V.natriegens | pPilin | 371.8 | 1 |
| 4 | V.natriegens | pPilin | 37.18 | 2 |
| 5 | DH5α | pGFP | 352.9 | 1 |
| 6 | DH5α | pGFP | 35.29 | 2 |
| 7 | V.natriegens | pGFP | 352.9 | 1 |
| 8 | V.natriegens | pGFP | 35.29 | 2 |
8.6
XIRAN HU
Colony PCR
The result of the overnight Agar plate cultures was as follows.There were several colonies on the plates corresponding to DH5α transformations but only one colony on transformation 7.
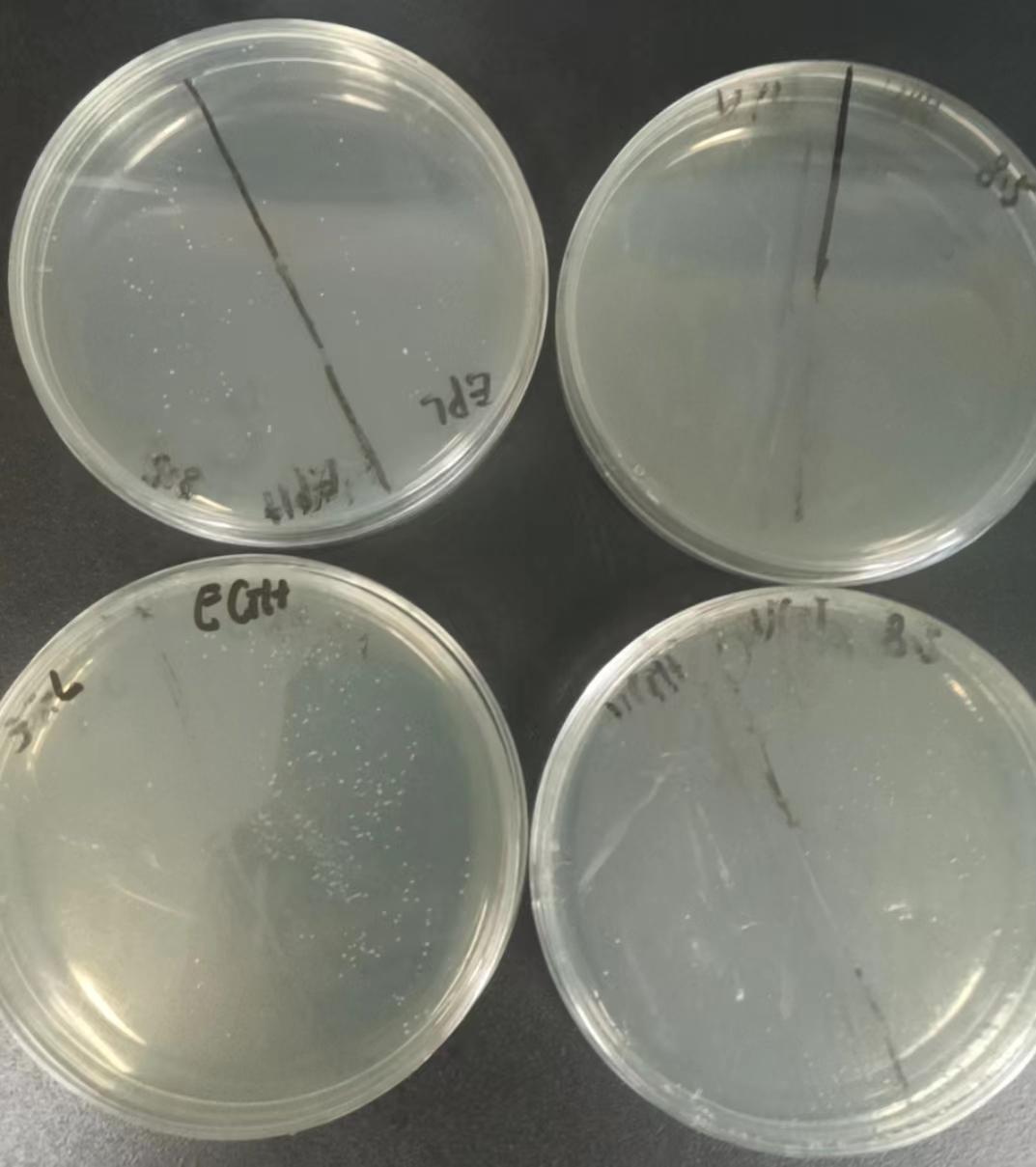
Several colonies were selected to conduct colony PCR with appropriate Primers.The result is as follows:
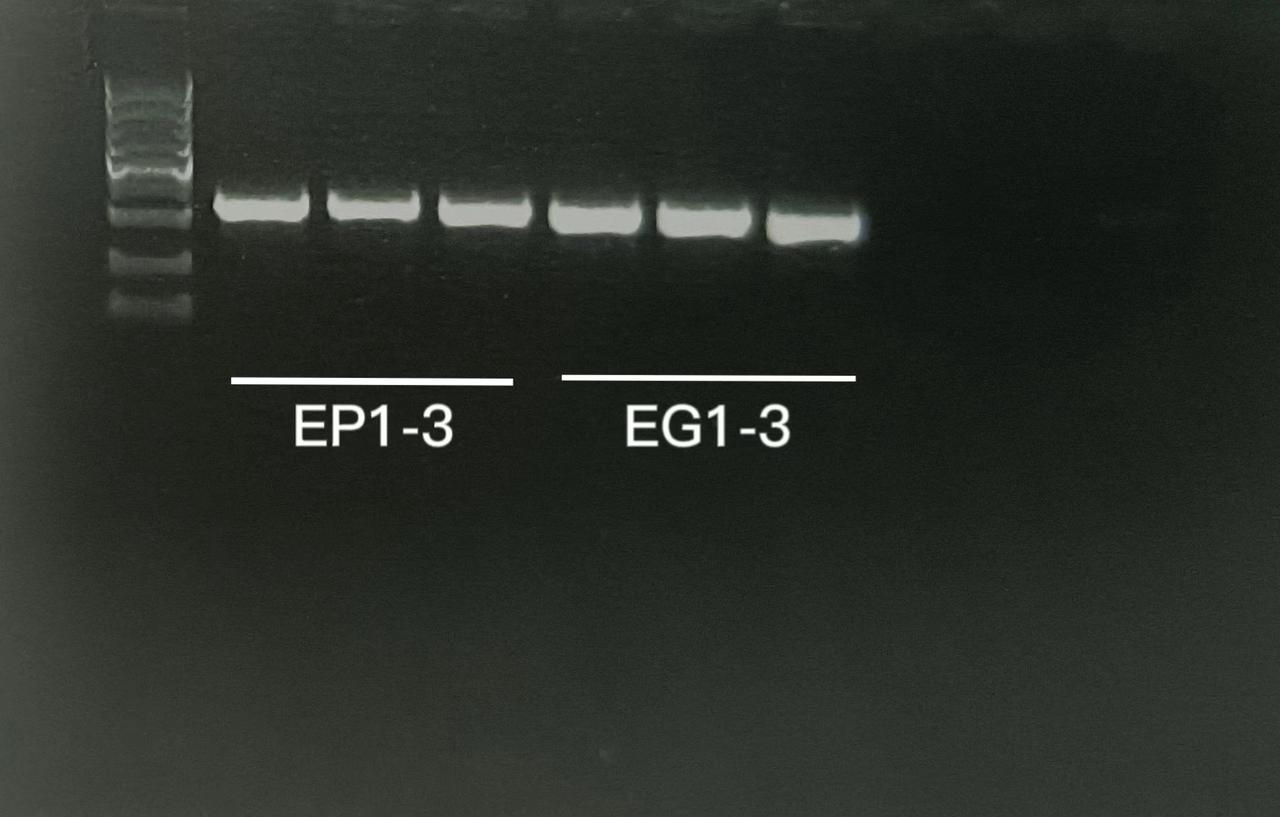
All positive strains were stored as glycerol stock. DH5α cells transformed with pPilin were designated as EP1,EP2,EP3 and DH5α cells transformed with pGFP were designated as EG1,EG2,EG3.
EP2 overnight culture
XIRAN HU
Strain EP2 was inoculated in 2mL of LB broth culture with 100μg/mL of Ampicilin for pilin induction.
Transformation of chemical competent cells
XIRAN HU
The transformation was performed on home-made chemical competent V.natriegens with the following parameters
number |
Cell type |
plasmid name |
Plasmid concentration(μg/μL) |
Plasmid quantity(μL) |
|---|---|---|---|---|
| 1 | V.natriegens | pGFP | 352.9 | 1 |
| 2 | V.natriegens | pGFP | 35.29 | 2 |
8.7
Colony PCR
YUCHENG ZHANG
There were 2 colonies on the plate corresponding to transformation 1. Colony PCR was conducted on these 2 colonies with appropriate Primers. But no band was seen.
EP2 extended culture and pilin induction
YUCHENG ZHANG
2×30 mL of LB broth with 100μg/mL of Ampicilin were inoculated with overnight EP2 liquid culture. Arabinose solution, the inducer, was added to one tube when OD600 of the liquid culture in it reached 0.5. And then the culture continued for 5h before harvest.
Protein extraction from EP2
ZIWEN XU
Soluable proteins were extracted and collected from EP2 after the induction.
Pilus harvest
XIRAN HU
Pilus were harvested according to the protocol from EP2 culture.
BCA test of soluable proteins extracted from EP2
XIRAN HU
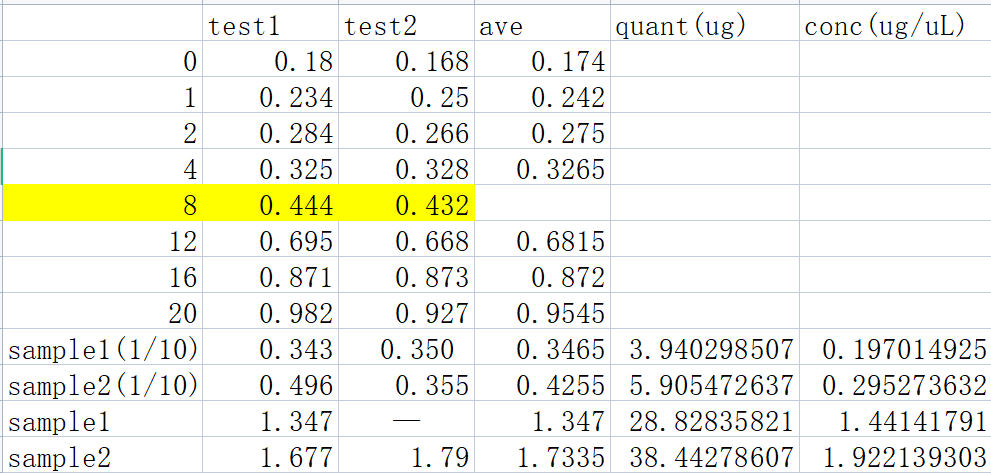
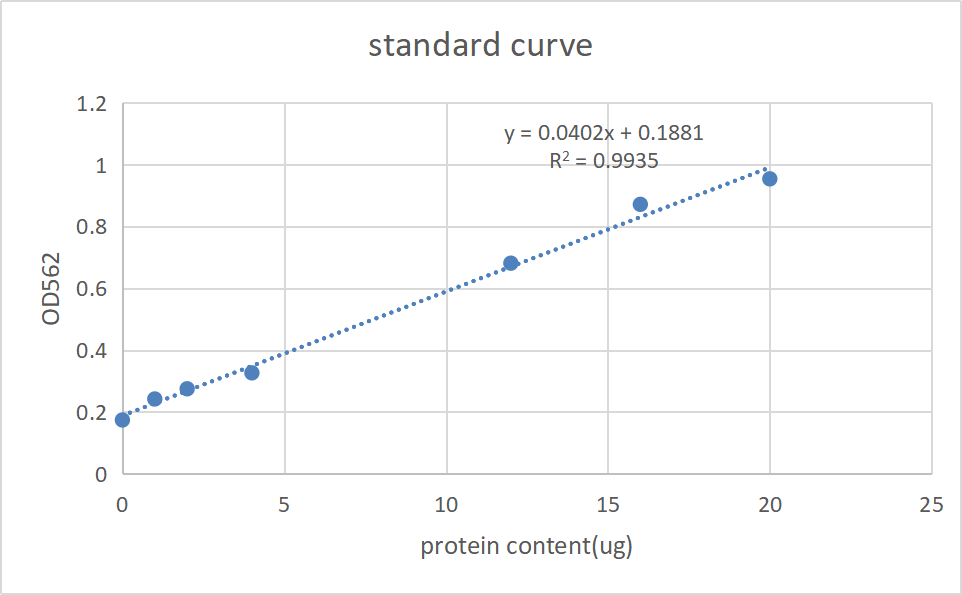
BCA test of soluable proteins extracted from EP2
8.9
ZIWEN XU
Whole cell lysates WB for Pilin identification
Overnight culture of EP2 was inoculated to the following culture mediums, 1.5mL for each condition:
Culturing time\induction concentration |
0 |
10mM |
20mM |
|---|---|---|---|
| 10h | 1 | 2 | 3 |
| 24h | 4 | 5 | 6 |
The induction of pilin expression was performed when the OD600 reached 0.5 by arabinose solution.
After stained with coomassie brilliant blue, no significant difference was seen between groups. The following steps were performed according to the protocols. No band was seen.
8.12
XIRAN HU
Glycerol stocks of the top 10 strains carrying plasmids ordered from Azenta were inoculated into liquid culture (LB broth with appropriate antibiotics). A glycerol stock of wild type Vibrio natriegens was inoculated into LB+v2 salt liquid medium for tomorrow's electrocompetent cells preparation.
8.13
Plasmid extraction
ZIWEN XU
Plasmids pGFP, pdCas9-sg1 and pPilin were extracted from Top 10 liquid culture. Concentrations and qualities of the plasmids were measured with Nanodrop.
pGFP |
pdCas9-sg1 |
pPilin |
|
|---|---|---|---|
| concentration(ng/μL) | 220.5 | 159.7 | 180.3 |
| OD260/280 | 1.82 | 1.88 | 1.88 |
| OD260/230 | 1.95 | 2.01 | 2.14 |
Ectrocompetent cells preparation(protocol 1)
YUCHENG ZHANG
Electrocompetent Vibrio natriegens were prepared as described in the protocol.
Electroporation
XIRAN HU
Electroporation was performed according to the protocol with the following parameters:
number |
voltage(v) |
time(ms) |
|---|---|---|
| 1 | 700 | 1 |
| 2 | 700 | 2 |
| 3 | 700 | 3 |
| 4 | 700 | 4 |
8.14
YUCHENG ZHANG
Electroporation result
There was no colony on the plates.
Electrocompetent cells preparation(protocol 1)
Electrocompetent Vibrio natriegens were prepared as described in the protocol.
8.15
XIRAN HU
Electroporation
Electroporation was performed according to the protocol with the following parameters:
number |
voltage(v) |
time(ms) |
|---|---|---|
| 1 | 700 | 1 |
| 2 | 700 | 2 |
| 3 | 800 | 1 |
| 4 | 800 | 1.7 |
| 5 | 820 | 1.8 |
| 6 | 900 | 1 |
Whole cell lysates WB for pilin identification
XIRAN HU
Overnight culture of EP2 was inoculated into the following mediums:
Number |
Inducer concentration |
Culturing time after induction |
|---|---|---|
| 1 | 0mM | 5h |
| 2 | 10mM | 5h |
| 3 | 20mM | 5h |
| 4 | 0mM | 15h |
| 5 | 10mM | 15h |
| 6 | 20mM | 15h |
After transfer, the PVDF membrane was stained with Ponceau S. No band was seen, perhaps due to the unstable current of the electrophoresis system.
8.17
XIRAN HU
Electroporation result
There was no colony on the plates.
electroporation
Electroporation was performed according to the protocol with the following parameters:
number |
voltage(v) |
time(ms) |
|---|---|---|
| 1 | 700 | 2.2 |
| 2 | 790 | 2.2 |
| 3 | 900 | 2.2 |
| 4 | 800 | 2.3 |
| 5 | 740 | 2.2 |
| 6 | 840 | 2.2 |
Transformation of chemical competent BL21(DE3) cells
ZIWEN XU
Comercial chemical competent BL21(DE3) cells were transformed with 3μL(684 ng) of pPilin according to the protocol.
8.18
Colony PCR
YUCHENG ZHANG
There were several colonies on the plate of BL21 transformation. Colony PCR was conducted on 4 of these colonies with appropriate Primers. The result was as follows.
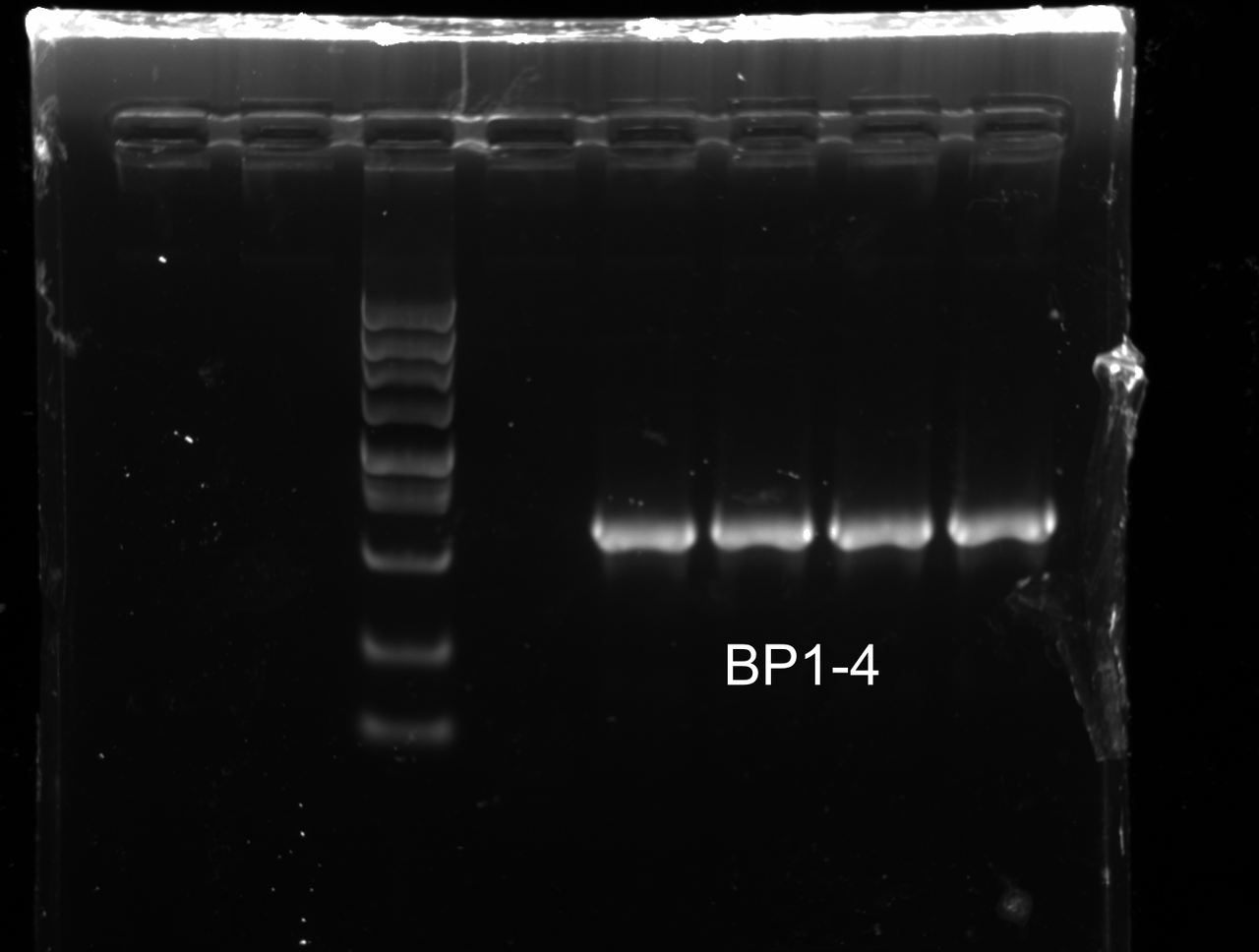
Strain corresponding to band 1 was designated as BP1.
BP2 extended culture and pilin induction
XIRAN HU
2×30 mL of LB broth with 100μg/mL of Ampicilin were inoculated with overnight EP2 liquid culture. Arabinose solution, the inducer, was added to one tube when OD600 of the liquid culture in it reached 0.5. And then the culture continued for 5h and 15h before harvest.
8.19
XIRAN HU
Whole cell lysates SDS-PAGE for pilin identification
Samples for SDS-PAGE were prepared from the following mediums:
number |
Inducer concentration |
Culturing time after induction |
|---|---|---|
| 1 | 0mM | 5h |
| 2 | 20mM | 5h |
| 3 | 0mM | 15h |
| 4 | 20mM | 15h |
No significant difference in the molecular weight of pilin was seen between the lanes.
Electrocompetent cell preparation (protocol 2)
YUCHENG ZHANG
Electrocompetent cells were prepared according to the protocol.
Electroporation
XIRAN HU
Electroporation was performed according to the protocol with the following parameters:
Plasmid |
Plasmid volume(μL) |
voltage(v) |
time(ms) |
|---|---|---|---|
| pPililn | 5 | 700 | 2.2 |
| pPilin | 5 | 790 | 2.2 |
| pGFP | 5 | 900 | 2.2 |
| pGFP | 5 | 800 | 2.3 |
| pdCas9-sg1 | 5 | 740 | 2.2 |
| pdCas9-sg1 | 5 | 840 | 2.2 |
8.20
YUCHENG ZHANG
Colony PCR
There were several colonies on the plates. Colony PCR was conducted on 11 of these colonies with appropriate primers. The result was as follows.
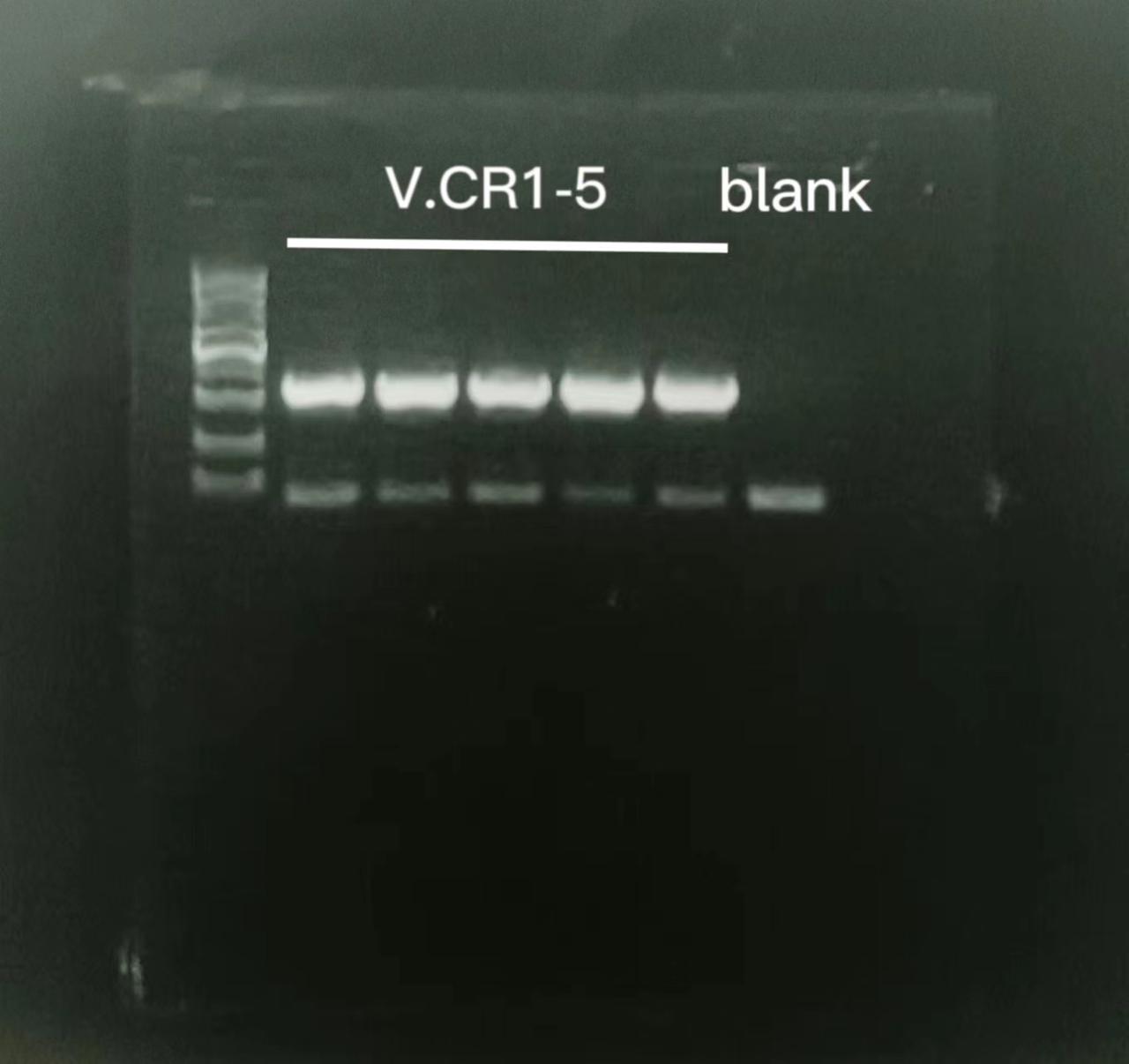
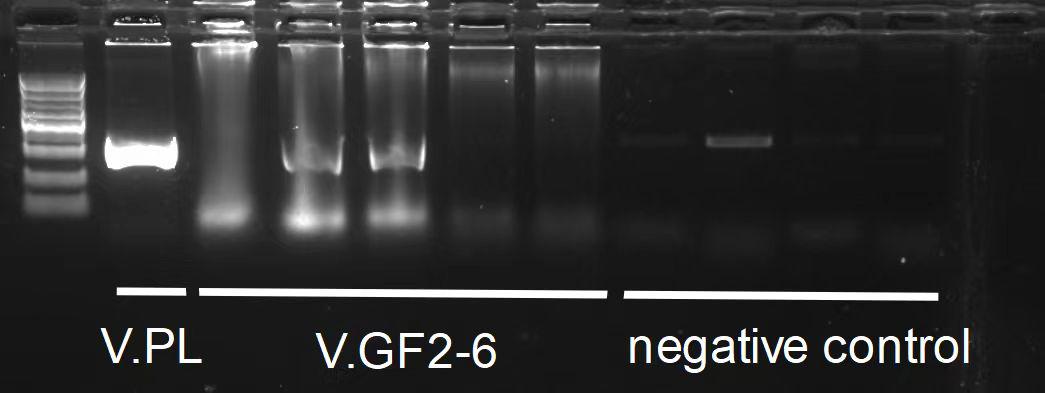
Strains tested positive were designated as V.CR1, V.CR2, V.CR3, V.CR4, V.CR5, V.PL V.GF3 and V.GF4.
8.21
V. CR1 extended culture and dCas9 induction
ZIWEN XU
Overnight V. CR1 culture was inoculated into the following mediums.Inducer was added when the OD600 reached 0.5.
number |
Inducer concentration(mg/mL) |
Culturing time after induction |
|---|---|---|
| 1 | 0 | 5h |
| 2 | 0.5 | 5h |
| 3 | 1 | 5h |
| 4 | 0 | 15h |
| 5 | 0.5 | 15h |
| 6 | 1 | 15h |
8.22
ZIWEN XU
Whole cell lysates SDS-PAGE for dCas9 identification
1 mL of each V.CR1 culture was centrfuged and the pellets were resuspended with 35 μL of ddH2O.
10 μL of 5×loading buffer was added to each of the tubes and the tubes were incubated at 95℃ for 5 min. 10 μL of prepared samples were loaded.
There was no significant difference in the molecular weight of dCas9 between lanes.
8.24
Green fluorescence observation
YUCHENG ZHANG
Overnight V.GF4 culture and Vibrio natriegens 14048 culture were enlarged for 2 hours.
The fluorescence was observed with fluorescence microscope. There was no significant difference between strain 14048 and V.GF4.
Pilin induction in V.PL
XIRAN HU
Overnight V.PL culture was inoculated into the following mediums at a dilution ratio of 1:100. Induction was performed 2h after the inoculation.
number |
inducer(arabinose) concentration |
Culturing time after induction |
Medium volume |
temperature |
|---|---|---|---|---|
| 1 | 0mM | 11h | 30mL | 37℃ |
| 2 | 0mM | 11h | 30m | 37℃ |
| 3 | 20mM | 11h | 30mL | 37℃ |
| 4 | 20mM | 11h | 30mL | 37℃ |
8.25
XIRAN HU
Pilus harvestion
Culture number 1 and 2 were consolidated while number 3 and 4 were consolidated. Pilus were harvested according to the protocol. The BCA test result of the two acquired samples was as follows.
Sample1 was from uninduced culture while sample2 was from induced culture. The final concentrations of the two samples were 0.39μg/μL and 0.28μg/μL, respectively.
1.5mL of sample1 and sample 2 were centrifuged to prepare whole cell lysate samples designated as sample1-1 ans sample2-1
Pilin WB
XIRAN HU
WB of pilin was performed on sample1, sample1-1, sample2 and sample2-1 according to the protocol.
8.26
WB result
XIRAN HU
There was no band on the membrane. The secondary antibody was not adequately washed off and small protein bands were too obscure to be observed. Tris-tricine SDS-PAGE should be used for pilin WB.
Extended culture of V.CR1 and dCas9 induction
ZIWEN XU
Overnight V.CR1 culture was inoculated into the following mediums. Inducer was added when the OD600 reached 0.5.
number |
Inducer concentration(mg/mL) |
Culturing time after induction |
|---|---|---|
| 1 | 0 | 6h |
| 2 | 0.5 | 6h |
Whole cell lysates SDS-PAGE for dCas9 identification
ZIWEN XU
1mL of each culture above was centrifuged and the pellets were resusppended with 35uL of ddH2O. 5uL of each of the prepared samples were loaded for SDS-PAGE. The result is as follows. The band marked with * was darker on the induced lane and the molecular weight of dCas9(about 150kDa) is between 180kDa and 130kDa.

8.27
Whole cell lysates SDS-PAGE for sfGFP identification
YUCHENG ZHANG
1 mL of overnight V.GF4 culture and 14048 culture were centrifuged and the pellets were resuppended with ddH2O. 5 μL of 5×loading buffer was added to the tubes which were then incubated at 95℃ for 5mins. 5 μL of each of the prepared samples was loaded for SDS-PAGE.
There were too many bands to distinguish the differences.
Electrocompetent cell preparation (protocol 2)
YUCHENG ZHANG
Overnight V.PL and V.CR1 culture were used for electro competent cell preparation according to the protocol.
Electroporation
XIRAN HU
Electroporation was performed according to the following parameters.
number |
Competent cell |
plasmid |
Plasmid volume(μL) |
time(ms) |
voltage(v) |
antibiotics |
|---|---|---|---|---|---|---|
| 1 | V.CR1 | pPilin | 5 | 4 | 790 | Ampicilin |
| 2 | V.CR1 | pPilin | 5 | 4 | 790 | Ampicilin |
| 3 | V.PL | pdCas9-sg1 | 5 | 3.8 | 790 | Kanamycin |
| 4 | V.PL | pdCas9-sg1 | 5 | 4 | 790 | Kanamycin |
8.28
V. CR1 extended culture and dCas9 induction
ZIWEN XU
Overnight V. CR1 and ATCC14048 culture was inoculated into the following mediums. Inducer was added when the OD600 reached 0.5.
number |
cell |
Inducer concentration(mg/mL) |
Culturing time after induction |
|---|---|---|---|
| 1 | WT | 0 | 5h |
| 2 | V.CR1 | 0 | 5h |
| 3 | V.CR1 | 0.5 | 5h |
| 4 | V.CR1 | 0.5 | 10h |
SDS-PAGE of dCas9
ZIWEN XU
Soluable proteins were extracted from 1.5 mL of the cultures above. The result of SDS-PAGE was as follows:
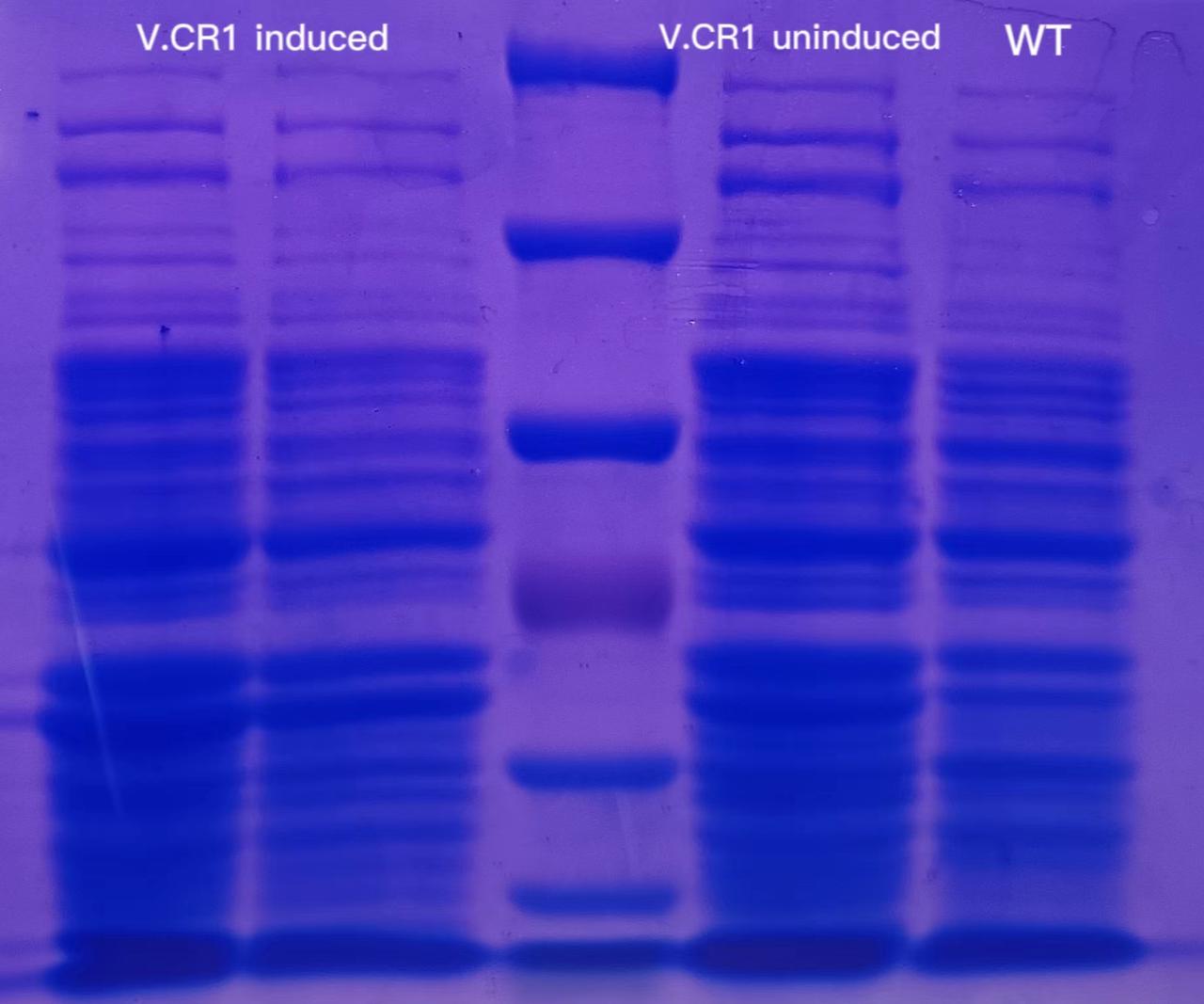
There was no significant difference between the 4 lanes.
SDS-PAGE of sfGFP
YUCHENG ZHANG
1 mL of overnight V.GF4 and ATCC14048 culture were centrifuged and then resuspended with 35 μL of ddH2O. 1.5 mL of overnight V.GF4 and ATCC14048 culture were used for soluable protein extraction. The result of SDS-PAGE was as follows.
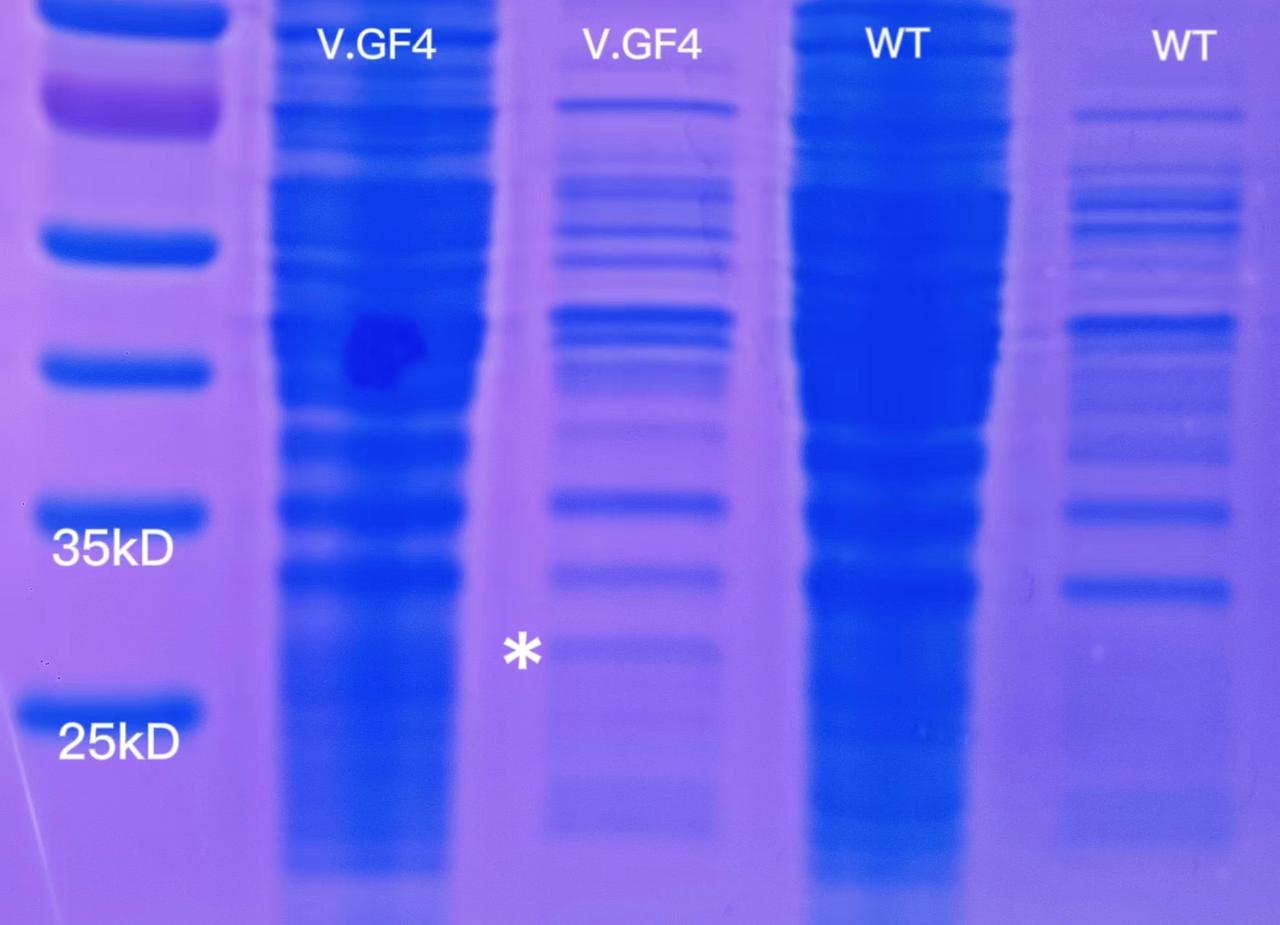
There was a significant darker band on the lane of V.GF4 soluable proteins(marked with).The molecular weight of the modified sfGFP is about 31kD,between 25kD and 35kD. The experiment would be repeated 2 times to ensure the result.
9.8
pGFP modification
YUCHENG ZHANG
Reversed PCR
Component
Volume
2 × Phanta Flash Master Mix
25μL
ddH2O
20μL
CutF+R(5μM)
4μL
pGFP
1μL
annealing temperature
59℃
elongation time
20sec
Gel extraction
The linearized pGFPcut was purified from PCR mix.
Gibson assembly
Component |
Volume |
|---|---|
| Linearized pGFP | 1 μL(333ng) |
| 2×CE mix | 5μL |
| ddH2O | 4μL |
The recombinant product (desingated as pGFPcut) was transformed into electrocompetent V.natriegens cells.
SDS-PAGE of sfGFP
XIRAN HU
1 mL of overnight V.GF4 and ATCC14048 culture were centrifuged and then resuspended with 35 μL ddH2O. 1.5mL overnight V.GF4 and ATCC14048 culture were used for soluable protein extraction. The result of SDS-PAGE was as follows.
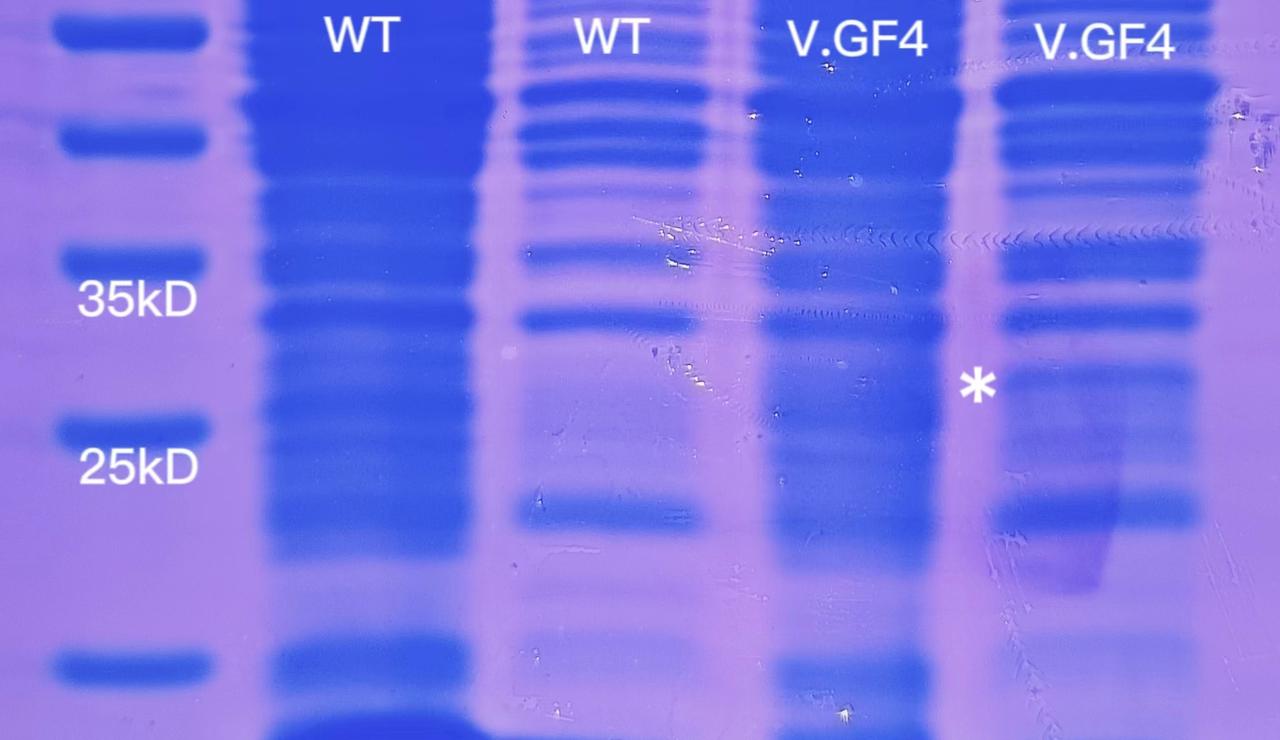
There was a significantly darker band in the lane of V.GF4 soluable proteins (marked with'*').The molecular weight of the modified sfGFP is about 31kD, between 25kD and 35kD. The experiment will be repeated one more time to confirm the result.
Electroporation
XIRAN HU
Electroporation was performed according to the following parameters.
number |
Competent cell |
plasmid |
Plasmid volume(μL) |
time(ms) |
voltage(v) |
antibiotics |
|---|---|---|---|---|---|---|
| 1 | V.CR1 | pPilin | 5 | 3.7 | 790 | Amp(40μg/mL) |
| 2 | V.PL | pdCas9-sg1 | 5 | 4 | 790 | Kan(50μg/mL) |
Pilin induction in V.PL
XIRAN HU
Overnight V.PL culture was inoculated into the following mediums at a dilution ratio of 1:100. Induction was performed 2h after the inoculation.
number |
Cell type |
inducer(arabinose) concentration |
Culturing time after induction |
Medium volume |
temperature |
|---|---|---|---|---|---|
| 1 | WT | 20mM | 16h | 30mL | 37℃ |
| 2 | V.PL | 20mM | 16h | 30m | 37℃ |
9.9
YUCHENG ZHANG
Colony PCR
There were several colonies on the plate supplemented with Kanamycin. 2 colonies were chosen to conduct colony PCR with the following parameters:
pPilin verification |
|
|---|---|
Component |
Volume |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| PLR+PLF(5μM) | 4μL |
| colony | 1 |
pdCas9-sg1 verification |
|
|---|---|
Component |
Volume |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| CRR+CRF(5μM) | 4μL |
| colony | 1 |
Bands were only seen on the lanes corresponding to pdCas9-sg1 verification. The electroporation was not successful.
SDS-PAGE of Pilin
XIRAN HU
1.5 mL of each of the 4 cultures were used for soluable protein extraction. 10μL of prepared sample was loaded. The result of SDS-PAGE was as follows. The issue may lie in the gel or loading buffer.
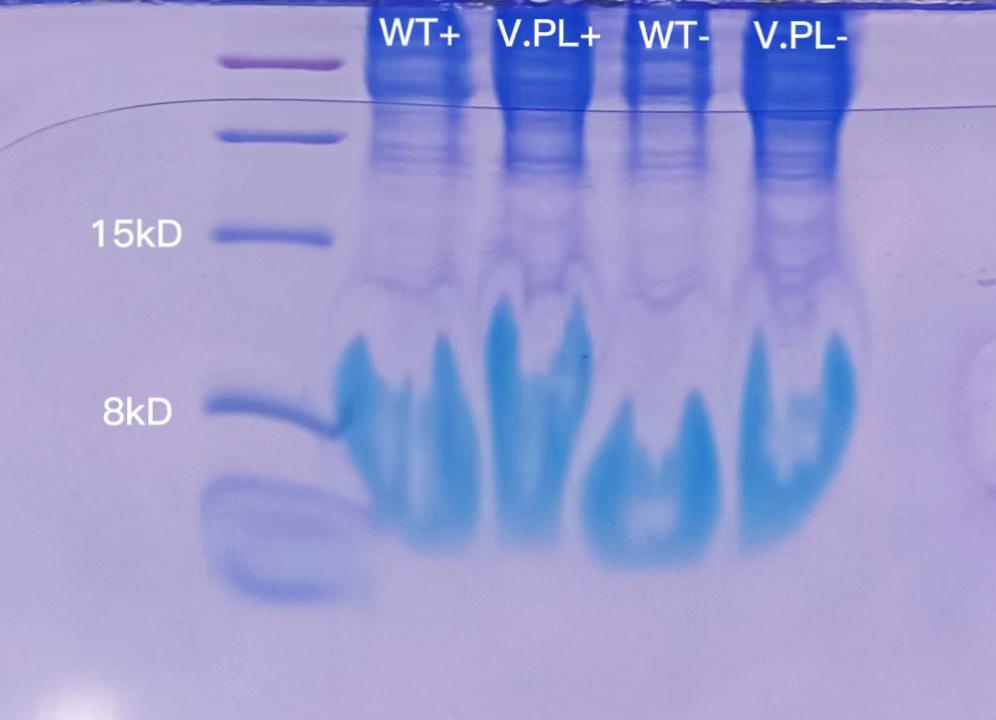
9.10
SDS-PAGE of sfGFP
YUCHENG ZHANG
1 mL of overnight V.GF4 and ATCC14048 culture were centrifugedand then resuspended with 35 μL ddH2O. 1.5mL of overnight V.GF4 and ATCC14048 culture were used for soluable protein extraction. The result of SDS-PAGE was as follows. a stands for all proteins and s stands for extracted soluable proteins.


There was a significant darker band on the lane of V.GF4 soluable proteins (marked with'*' ).The molecular weight of the modified sfGFP is about 31kD,between 25kD and 35kD.
Gibson assembly
ZIWEN XU
The linearized pGFP solution was diluted to 67.26 μg/mL.
Component |
Volume |
|---|---|
| Linearized pGFP(diluted) | 4 μL |
| 2×CE mix | 5 μL |
| ddH2O | 1 μL |
Chemical and electrocompetent cell prepareation (protocol 2)
YUCHENG ZHANG
Electroporation competent V.PL and V.CR1 were prepared according to the protocols.
Electroporation
Electroporation was performed according to the protocol with the following parameters.
Cell type |
plasmid |
Plasmid volume(μL) |
voltage(v) |
time(ms) |
|---|---|---|---|---|
| V.CR1 | pPilin | 5 | 790 | 4 |
| V.CR1 | pGFPcut | 5 | 790 | 3.9 |
| V.PL | pdCas9-sg1 | 5 | 790 | 4 |
Transformation of chemical competent DH5α
ZIWEN XU
2 μL of modified pET-24b(+) was added to 50 μL of chemical competent DH5α for transformation.
Pilin induction in V.PL
XIRAN HU
Overnight V.PL culture was inoculated into the following mediums at a dilution ratio of 1:100. Induction was performed 2h after the inoculation.
number |
Cell type |
inducer(arabinose) concentration |
Culturing time after induction |
Medium volume |
temperature |
|---|---|---|---|---|---|
| 1 | WT | 20mM | 20h | 2×30mL | 16℃ |
| 2 | V.PL | 20mM | 20h | 2×30mL | 16℃ |
9.11
XIRAN HU
Modified pET-24(+) carrying strain storage.
There was no colony on the plates of electroporation, maybe due to the overly used electroporation cuvettes. There were many colonies on the plate of DH5α transformation and 5 colonies were chosen to be strored.
Pilus harvest and SDS-PAGE of pilin
XIRAN HU
Pilus were harvested according to the protocol. The BCA test result of the harvested pilus solution was as follows.
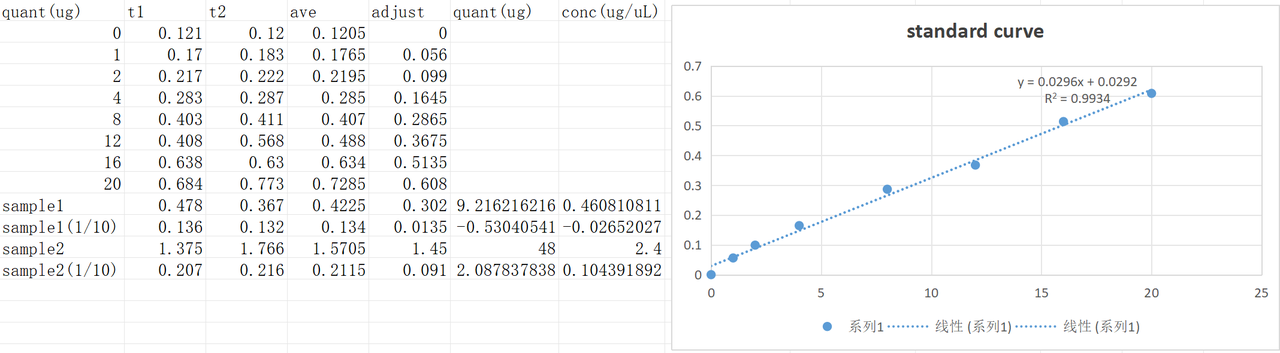
According to the result, the concentration of sample1 (induced V.PL) was 0.46μg/μL and the concentration of sample2(WT) was 1.04μg/μL.
Electroporation
YICHEN LIU
Electroporation was performed according to the protocol with the following parameters.
Cell type |
plasmid |
Plasmid volume(μL) |
voltage(v) |
time(ms) |
|---|---|---|---|---|
| V.CR1 | pPilin | 5 | 790 | 4 |
| V.PL | pdCas9-sg1 | 5 | 790 | 4 |
9.12
Colony PCR
XIRAN HU
5 colonies on the plates were chosen for colony PCR with the following parameters
pPilin verification |
|
|---|---|
Component |
Volume |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| PLR+PLF(5μM) | 4μL |
| colony | 1 |
pdCas9-sg1 verification |
|
|---|---|
Component |
Volume |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| CRR+CRF(5μM) | 4μL |
| colony | 1 |
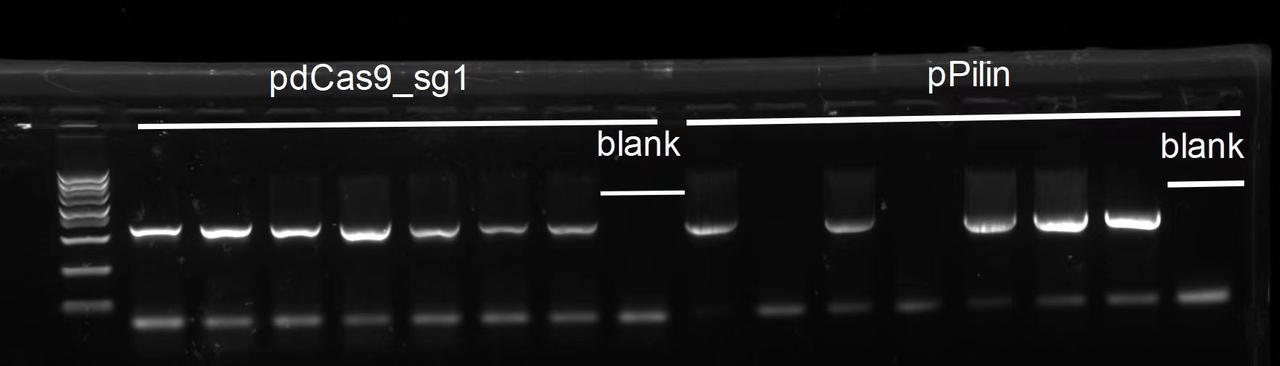
The result was as follows. Strains that had bands in both PCR tests were stored as glycerol stock and designated as V.PC1, V.PC3, V.PC5, V.PC6 and V.PC7, respectively.
WB of harvested pilin
XIRAN HU
Step |
Details |
|---|---|
| blocking | 5% silk milk dissolved in TBST, 1.5h at RT |
| Primary antibody | 1:4000, overnight at 4℃ |
| Secondary antibody | 1:5000, 1h at RT |
9.13
WB of harvested pilin
The result of the WB was as follows. Marker bands of low molecular weight could not be seen, maybe because the transfer buffer was not supplemented with 20% of methanol.

Plasmid exraction
XIRAN HU
Plasmids were extracted from overnight culture of V.PC1,V.PC3,V.PC5,V.PC6,V.PC7 for sequencing.
Electroporation
YICHEN LIU
number |
Cell type |
plasmid |
Plasmid volume |
voltage |
time |
|---|---|---|---|---|---|
| 1 | V.CR1 | pGFPcut | 5 μL | 790v | 4ms |
| 2 | ATCC14048 | Control plasmid | 5 μL | 790v | 4ms |
V.CR1 extended culture and dCas9 induction
ZIWEN XU
Overnight V.CR1 culture was inoculated into the following mediums. Inducer was added when the OD600 reached 0.5.
number |
Cell type |
Inducer concentration(mg/mL) |
Culturing time after induction |
temperature |
|---|---|---|---|---|
| 1 | WT | 0 | 20h | 20 ℃ |
| 2 | V.CR1 | 0.5 | 20h | 20℃ |
9.15
pGFP modification
YUCHENG ZHANG
Reversed PCR |
|
|---|---|
Component |
Volume/Details |
| 2 × Phanta Flash Master Mix | 25μL |
| ddH2O | 20 μL |
| modF1+R1(5μM) | 4 μL |
| pGFP | 1 μL |
| annealing temperature | 53℃ |
| elongation time | 21sec |
Gel extraction:Linearized pGFPmod1 was purified from PCR mix.
Gibson assembly |
|
|---|---|
Component |
Volume/Details |
| Linearized pGFP | 2 μL(256ng) |
| 2×CE mix | 5 μL |
| ddH2O | 3 μL |
The recombinant product (desingated as pGFPmod1) was transformed into chemical competent DH5α cells according to the protocol.
Pilin induction in V.PC6
XIRAN HU
Overnight V.PC6 culture was inoculated into the following mediums at a dilution ratio of 1:100. Induction was performed 2h after inoculation.
Parameter |
Details |
|---|---|
| Cell type | V.PC6 |
| inducer(arabinose) concentration | 20mM |
| Culturing time after induction | 24h |
| Medium volume | 2×200 mL |
| temperature | 20℃ |
SDS-PAGE of dCas9
ZIWEN XU
1 mL of induced V.CR1 and ATCC14048 culture were centrifuged and then resuspended with 35 μL of ddH2O. 1.5mL of overnight V.GF4 and ATCC14048 culture were used for soluable protein extraction. The result of SDS-PAGE was as follows. There was no significant difference between the two lanes.
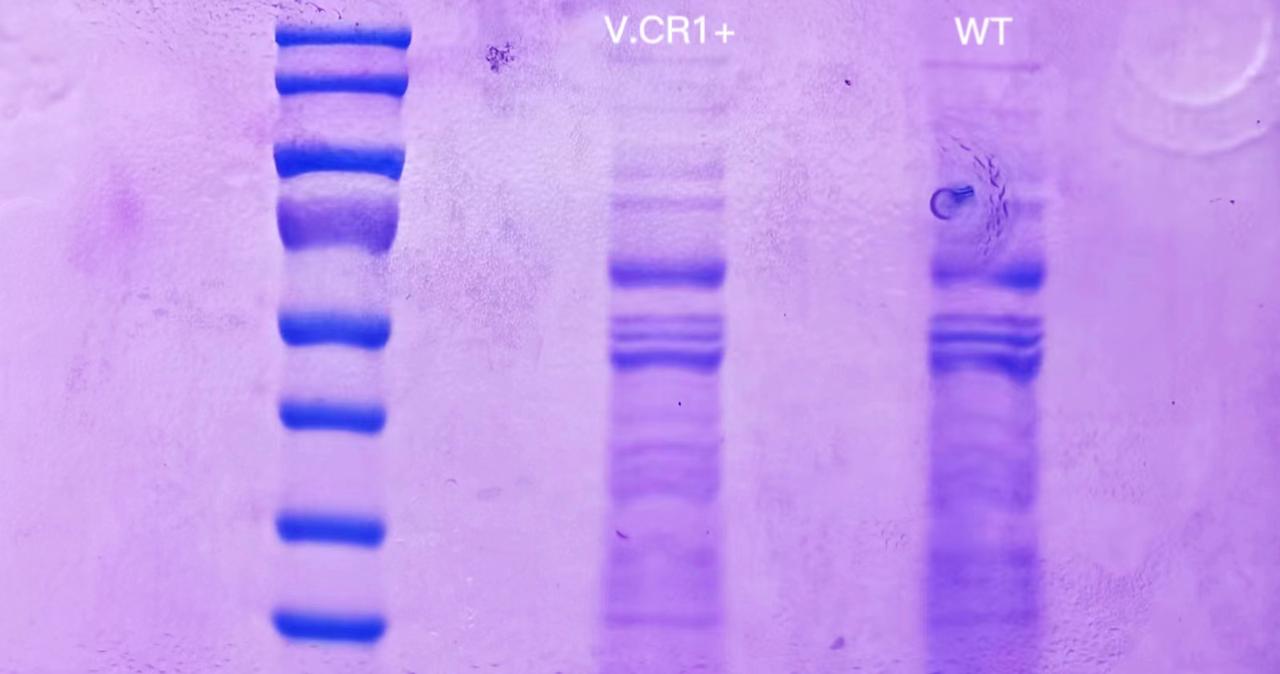
9.16
Pilus harvest
XIRAN HU
Pilus were harvested according to the protocol. The BCA test result of the harvested pilus solutions was as follows. Concentration was 4.92μg/μL of sample1 ,4.62μg/μL of sample2, and 0.88μg/μL of sample3.
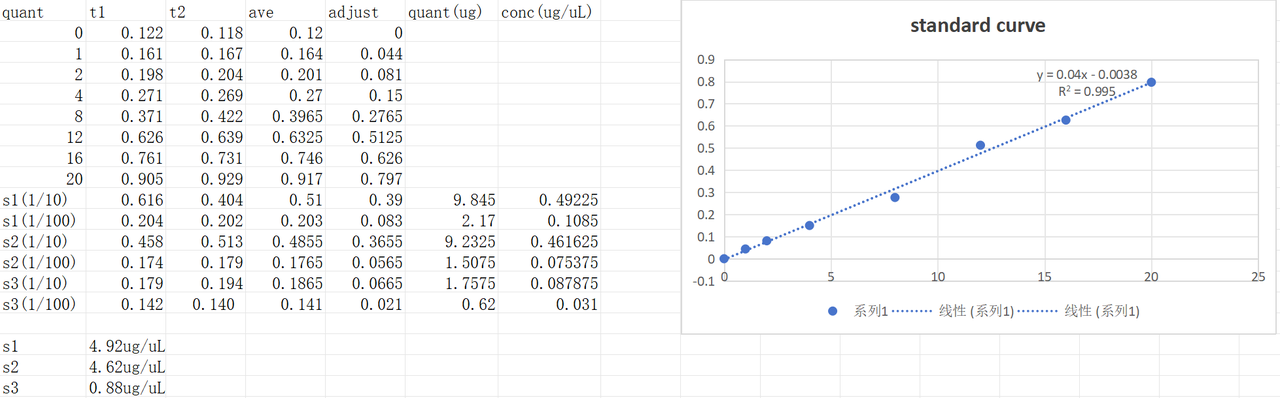
Chemical competent DH5α preparation
YUCHENG ZHANG
Chemical competent DH5α was prepared according to the protocols.
DH5α transformation
ZIWEN XU
Recombinant pGFPmod1 was transferomed into chemical competent DH5α cells accroding to the protocol.
9.17
XIRAN HU
WB of pilus
10 μL 2×tricine loading buffer was added to 40 μL of sample1 and sample2 respectively. Then the tubes were incubated at 95℃ for 5mins. 20.3μL of prepared sample1 and 21.8μL of prepared sample2 were loaded for SDS-PAGE. The primary antibody was diluted at 1:500.
Colony PCR
YUCHENG ZHANG
7 colonies on the plates were chosen for colony PCR with the following parameters
pPilin verification |
|
|---|---|
Component |
Volume/Details |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| GFR+GFF(5μM) | 4μL |
| colony | 1 |
The result was as follows.All colonies tested positive. Strains corresponding to band 1 and band 2 were chosen to conduct the following experiments and were designated as V.mod1-1 and V.mod1-2.
Pilin induction in V.PC6
XIRAN HU
Overnight V.PL culture was inoculated into the following mediums at a dilution ratio of 1:100. Induction was performed 2h after inoculation.
Cell type |
V.PC6 |
ATCC14048 |
|---|---|---|
| inducer(arabinose) concentration | 20mM | 20mM |
| Culturing time after induction | 24h | 24h |
| Medium volume | 100 mL | 100mL |
| temperature | 20℃ | 20℃ |
9.18
WB of pilus
XIRAN HU
The secondary antibody was diluted at 1:5000. No band was seen on the membrane.
Gibson assembly and DH5α transformation
YICHENG ZHANG, ZIWEN XU
gibson assembly
Component |
Volume/Details |
|---|---|
| Linearized pGFP | 2 μL(256ng) |
| 2×CE mix | 5 μL |
| ddH2O | 3 μL |
The recombinant product (desingated as pGFPcut) was transformed into chemical competent DH5α cells according to the protocol.
9.19
Plasmid extraction
ZIWEN XU
Plasmids were extracted from overnight culture of V.mod1-1 and V.mod1-2.
Pilus harvest and SDS-PAGE of pilin
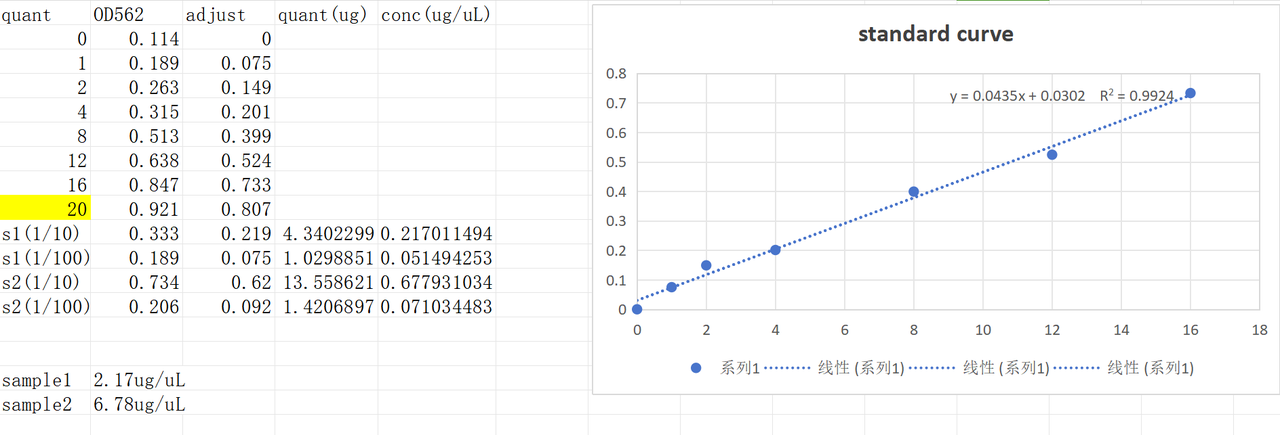
Pilus were harvested according to the protocol. The BCA test result was as follows. Sample 1 was harvested from V.PC6 culture and sample 2 was harvested from ATCC14048 culture.
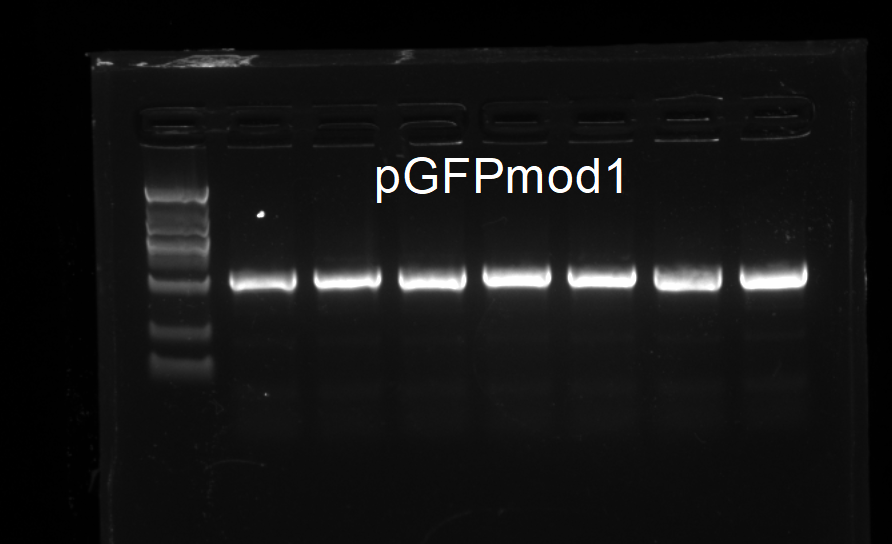
Colony PCR
YUCHENG ZHANG
One colony on the plates was chosen for colony PCR with the following parameters.
pPilin verification |
|
|---|---|
Component |
Volume/Details |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| GFR+GFF(5μM) | 4μL |
| colony | 1 |
The result was as follows.The colony tested positive and was designated as V.cut.
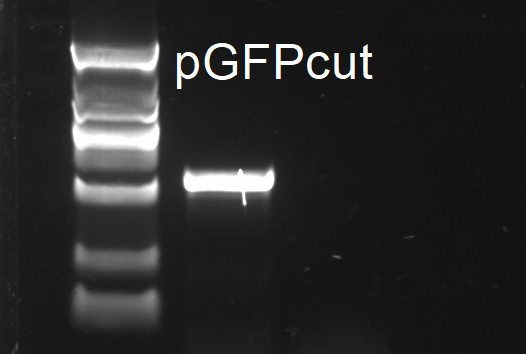
pGFP modification
YUCHENG ZHANG
Reversed PCR
PCR Parameters |
|
|---|---|
Component |
Volume/Details |
| 2 × Phanta Flash Master Mix | 25μL |
| ddH2O | 20 μL |
| modF2+R2(5μM) | 4 μL |
| pGFPmod1 | 1 μL |
| annealing temperature | 56℃ |
| elongation time | 21sec |
Linearized pGFPmod2 was purified from PCR mix.
Gibson assembly
Component |
Volume/Details |
|---|---|
| Linearized pGFPmod1 | 2 μL(256ng) |
| 2×CE mix | 5 μL |
| ddH2O | 3 μL |
The recombinant product (desingated as pGFPmod2) was transformed into chemical competent DH5α cells according to the protocol.
DH5α transformation
ZIWEN XU
Recombinant pGFPmod2 was transferomed into chemical competent DH5α cells accroding to the protocol.
9.20
YUCHENG ZHANG
Chemical competent DH5α preparation
YUCHENG ZHANG
Chemical competent DH5α was prepared according to the protocols.
Plamisd extraction
XIRAN HU
Plamis was extracted from overnight culture of V.cut
DH5α transformation
XIRAN HU
Recombinant pGFPmod2 was transferomed into chemical competent DH5α cells accroding to the protocol.
9.21
Colony PCR
YUCHENG ZHANG
Five colonies on the plates was chosen for colony PCR with the following parameters.
pPilin verification |
|
|---|---|
Component |
Volume/Details |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| GFR+GFF(5μM) | 4μL |
| colony | 1 |
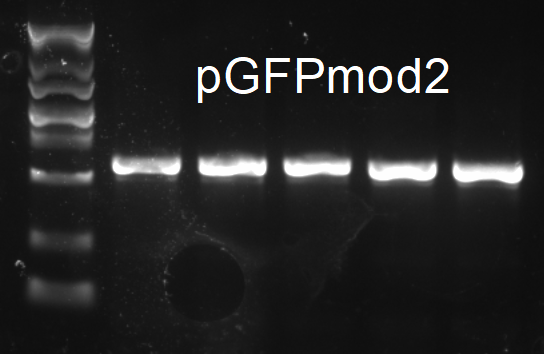
The result was as follows.One of the colonies tested positive was chosen for the following experiments.
Spread plate
XIRAN HU
Overnight V.PC1 culture was spread out on twenty agar plates supplement with kanacymin, ampicillin,0.5% glycerol and 2% arabinose. 300μL of culture was spread for each plate.The plates were cultured at 37℃ for 24 h before pilus harvest.
Electroporation
XIRAN HU
Cell type |
ATCC14048 |
|---|---|
| plasmid | pGFPcut |
| Plasmid volume | 5 μL |
| voltage | 790 v |
| time | 4 ms |
9.22
Electroporation
YUCHENG ZHANG
no. |
Cell type |
plasmid |
Plasmid volume |
voltage |
time |
|---|---|---|---|---|---|
| 1 | V.CR1 | pGFPcut | 5 μL | 790 v | 4 ms |
| 2 | V.CR1 | pGFPmod2 | 5 μL | 790 v | 4 ms |
Pilus harvest
XIRAN HU
The cells were harvested from the plates with 1 mL of LB+v2 salts medium for each plate.The following procedures were the same as the previous harvest protocol.
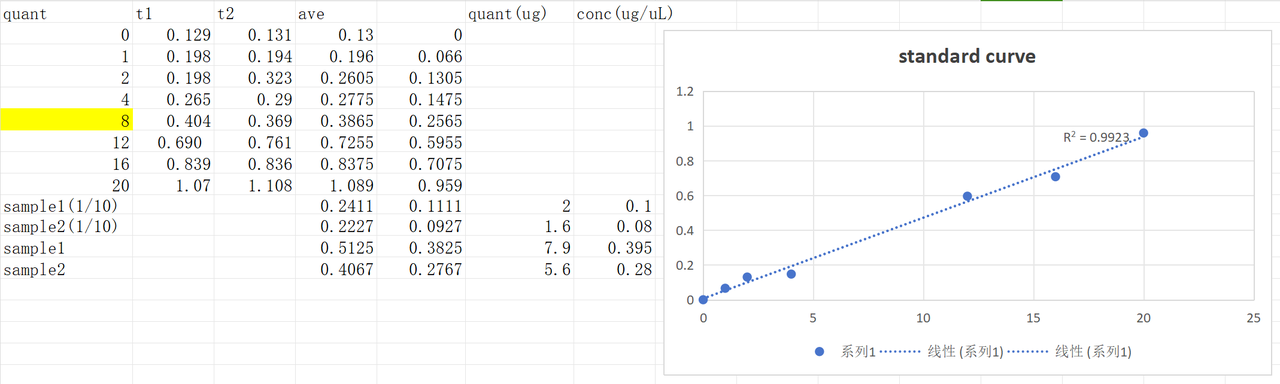
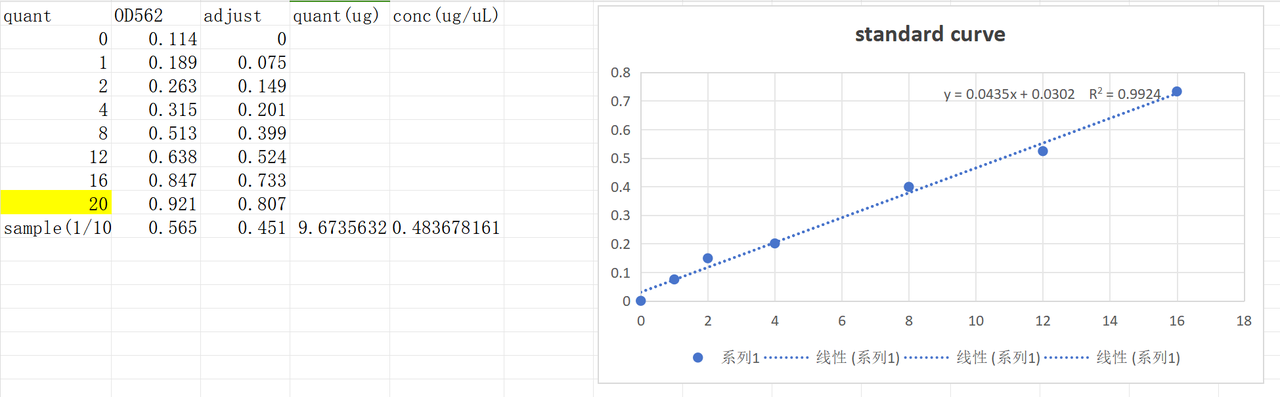
The concentration of harvested pilus solution was tested using the previous standard curve.The concentration of original pilus solution was about 4.8μg/μL
9.23
Electroporation
YICHEN LIU
no. |
Cell type |
plasmid |
Plasmid volume |
voltage |
time |
|---|---|---|---|---|---|
| 1 | V.CR1 | pGFPcut | 5 μL | 790 v | 4 ms |
| 2 | V.CR1 | pGFPmod2 | 5 μL | 790 v | 4 ms |
electrocompetent cell prepareation (protocol 2)
YUCHENG ZHANG
Electroporation competent V.CR1 were prepared according to the protocols.
9.24
Colony PCR
Six colonies from the plate of electroporation no.1 and five from no.2 were chosen for colony PCR.
pPilin verification |
|
|---|---|
Component |
Volume/Details |
| 2 × Taq Master Mix | 25μL |
| ddH2O | 20μL |
| GFR+GFF(5μM) | 4μL |
| colony | 1 |
The result is as follows. Two strains(designated as V.CR-cut and V.CR-mod respectively) were chosen to perform the following experiments.
Spread plate
ZIWEN XU
Overnight V.PC1 culture was spread out on twenty agar plates supplement with kanacymin, ampicillin,0.5% glycerol and 2% arabinose. 300μL of culture was spread for each plate.The plates were cultured at 37℃ for 24 h before pilus harvest.
electrocompetent cell prepareation (protocol 2)
YUCHENG ZHANG
Electroporation competent ATCC14048 were prepared according to the protocols.
9.25
Electroporation
XIRAN HU
no. |
Cell type |
plasmid |
Plasmid volume |
voltage |
time |
|---|---|---|---|---|---|
| 1 | ATCC14048 | pGFPcut | 5 μL | 790 v | 3 ms |
| 2 | ATCC14048 | pGFPmod2 | 5 μL | 790 v | 3 ms |
Green fluorescence measurement
XIRAN HU
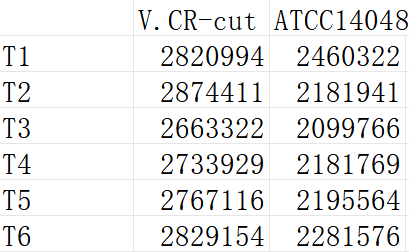
Overnight culture of V.CR-cut and V.CR were used for green fluorescence measurement with microplate reader.The excitation light was 488 nm and emitted light was 535 nm.the The result was as follows.
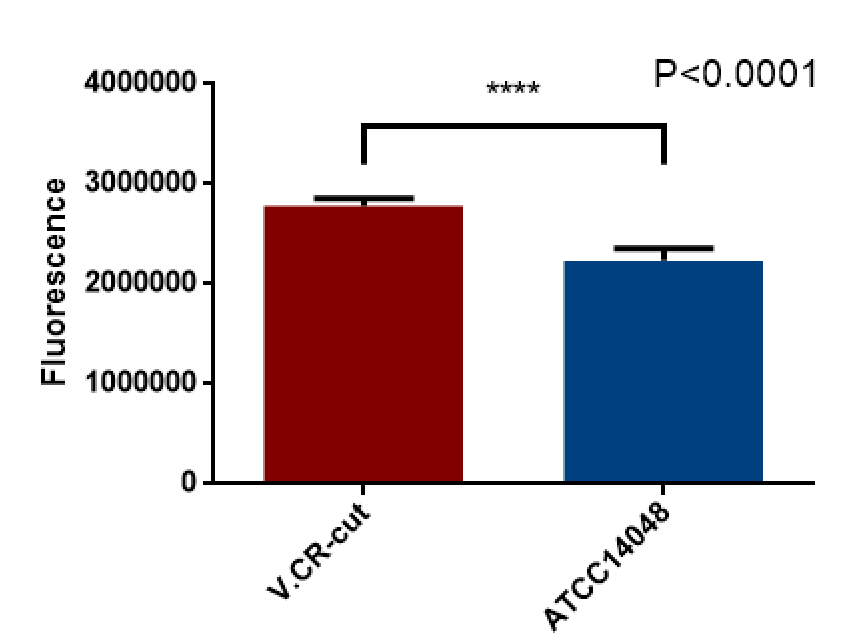
9.26
Pilus harvest
XIRAN HU
The cells were harvested from the plates with 1 mL of LB+v2 salts medium for each plate.The following procedures were the same as the previous harvest protocol.The BCA test result was as follows.The concentration of harvested pilin solution was 4.42 μg/μL.
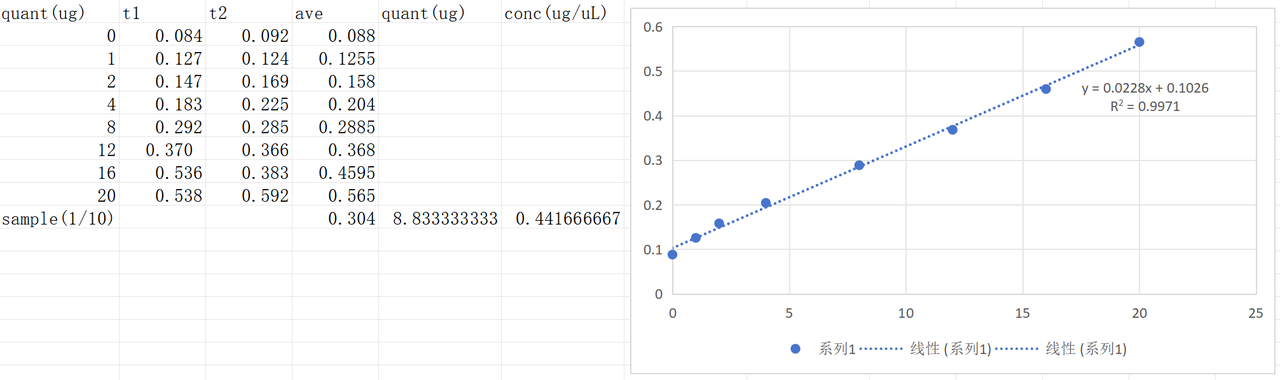
Sample preparation for TEM
XIRAN HU
The pilin solution was seriously diluted. 5 μL of each sample was dropped on 200-mesh carbon-coated copper
grids and let air-dry for 2 h.Then the samples were stained by uranium acetate and dried at 4 ℃ overnight.
Colony PCR
XIRAN HU
Five colonies from the plate of electroporation no.1 and two from no.2 were chosen for colony PCR.
The result is as follows. Two strains(designated as V.cut and V.mod respectively) were chosen to perform the following experiments.
9.27
YICHEN LIU, YUCHENG ZHANG
Transmission electron microscopy
The result of TEM was as follows.There was too much impurity in our sample, so we could not acquire the structure of our pilus film.
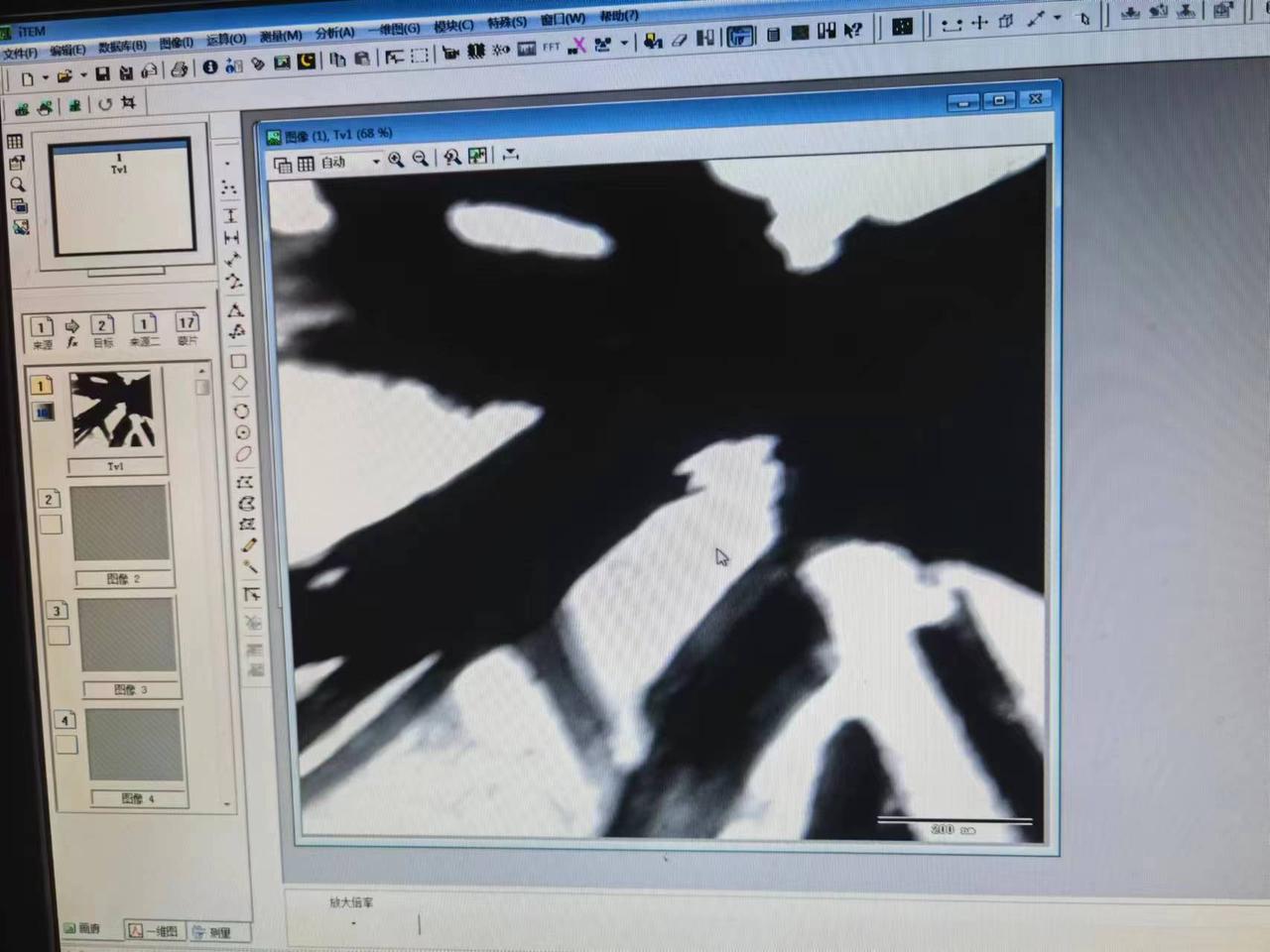
9.28
Spread plate
ZIWEN XU
Overnight V.PC1 culture was spread out on twenty agar plates supplement with kanacymin, ampicillin,0.5% glycerol and 2% arabinose. 300μL of culture was spread for each plate.The plates were cultured at 30℃ for 24 h before pilus harvest.
9.29
Spread plate
ZIWEN XU
Overnight culture of V.cut and V.mod was spread out on agar plates supplement with Ampicillin and cultured for 24 h before harvest to test the fluorscence intensity.
9.30
Pilus harvest and purification
XIRAN HU, ZIWEN XU
The cells were harvested from the plates with 1 mL of LB+v2 salts medium for each plate.The following procedures were the same as the previous harvest protocol(protocol 2). The harvested pilus solution was purified by Ni-NTA resin.
SDS-PAGE of pilin
ZIWEN XU
15 μL of washing 4 and elution 1 and elution 2 were loaded for SDS-PAGE. No obvious bands could be seen ,maybe because the duratio of second ammonium sulfate precipiation was too short.

pdCas9-sg modification
YUCHENG ZHANG
Component |
Volume/Concentration |
|---|---|
| 2 × Phanta Flash Master Mix | 25uL |
| ddH2O | 20uL |
| repF+R(5 μM) | 4uL |
| pdCas9-sg | 1uL |
| annealing temperature | 59℃ |
| elongation time | 40 sec |
Gel extraction
Linearized pGFPmod1 was purified from PCR mix.
Gibson assembly
Component |
Volume/Concentration |
|---|---|
| Linearized pdCas9-sg | 2 μL(256ng) |
| 2×CE mix | 5uL |
| ddH2O | 3uL |
The recombinant products (desingated as pdCas9-sg2,pdCas9-sg3 and pdCas9-sg4) were transformed into chemical competent DH5α cells according to the protocol.
10.1
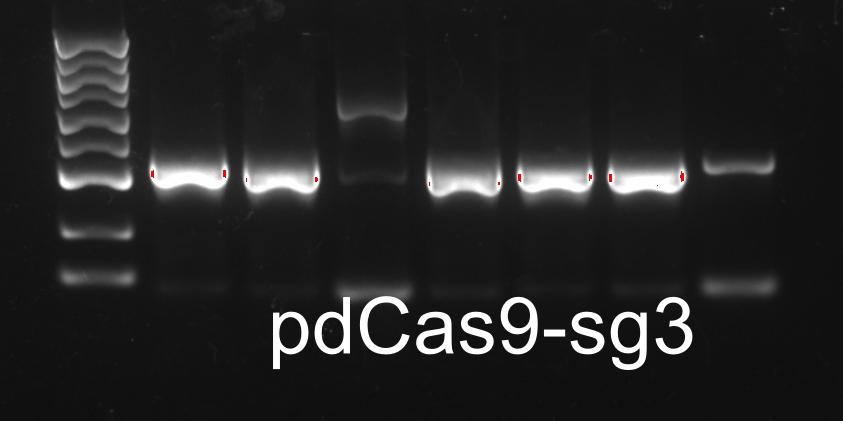
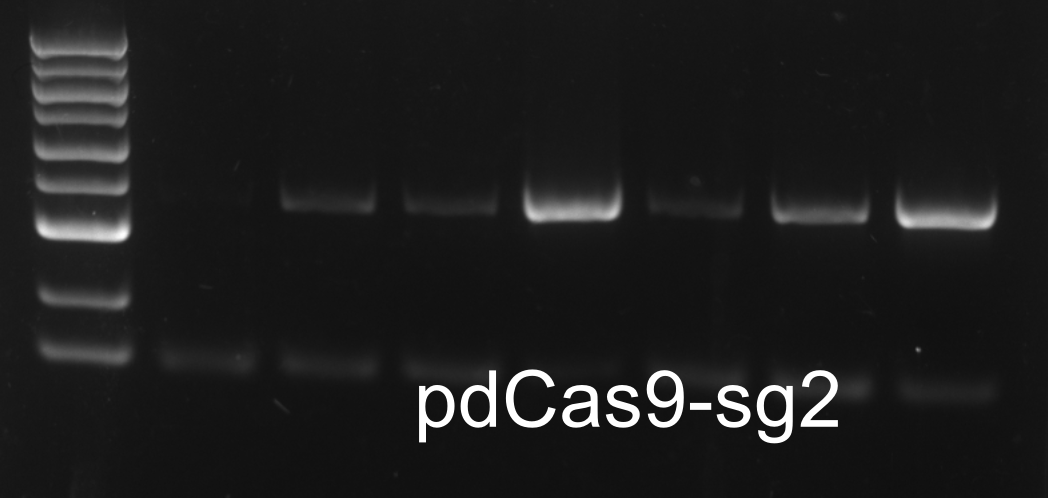
Colony PCR
XIRAN HU
Colonies from the plates of DH5α transfromed with modified pdCas9-ga were chosen for colony PCR. The result was as follows.One colony from each plate(designated as E.sg2, E.sg3 and E.sg4 respectively.) were chosen for the following experiments.
pPilin verification |
Volume/Concentration |
|---|---|
| 2 × Taq Master Mix | 25uL |
| ddH2O | 20uL |
| CRR+CRF(5uM) | 4uL |
| colony | 1 |
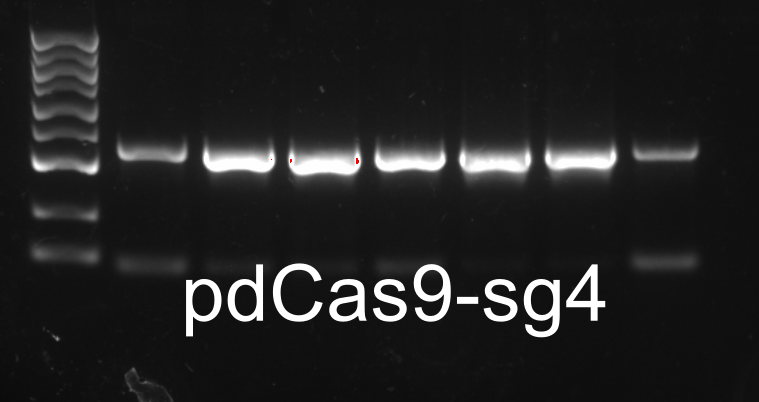
Spread plates
ZIWEN XU
Overnight V.PC1 culture was spread out on twenty agar plates supplement with kanacymin, ampicillin,0.5% glycerol and 2% arabinose. 300uL of culture was spread for each plate.The plates were cultured at 30℃ for 48 h before pilus harvest.
10.3
Pilus harvest and purification
XIRAN HU
The cells were harvested from the plates with 1 mL of LB+v2 salts medium for each plate.The following procedures were the same as the previous harvest protocol(protocol 2). The harvested pilus solution was purified by Ni-NTA resin.
10.6
SDS-PAGE of pili
XIRAN HU
10 μL of all washing and elution solution were loaded for SDS-PAGE. The result was as follows.

