The general tryptophan repressor (TrpR) is not specific, and we wanted to obtain mutant that could stably suppress tryptophan (Trp) expression. Therefore, we apply the compartmentalized partnered replication (CPR) to perform positive and negative selection. Each one positive or negative selection is called a cycle (Figure 3).
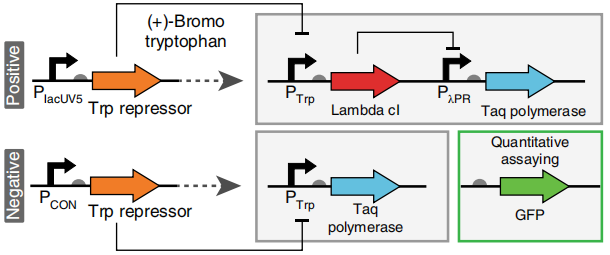
In vivo circuit diagrams used for positive
and negative CPR selections.
The tryptophan repressor (TrpR) undergoes transcription, translation and dimerization to
eventually form a dimer. The dimeric form of the tryptophan repressor (TrpR) has one Trp
or Br-Trp binding site per monomer, each binding to a Trp or Br-Trp (Figure 4).
In the positive selection environment, only tryptophan but not bromotryptophan is present.
If TrpR is the repressor that we want to bind strongly with the tryptophan (Trp), then it is
possible to inhibit the expression of the Lamda repressor after binding with tryptophan. The role of the Lamda repressor is to inhibit the expression of Taq polymerase. Hence, as Lambda repressor is inhibited, Taq expression will increase. To measure the amount of Taq polmerase, e construct pNEG- sfGFP and pPOS-sfGFP which replace Taq polymerase gene with sfGFP to characterize the expression of Taq polymerase.
In the negative screening environment, symmetrical to the positive screening, only bromotryptophan (Br-Trp) is present without tryptophan (Trp). If TrpR is the repressor we want that binds weakly to the bromotryptophan (Br-Trp), it does not inhibit Taq expression, allowing the increase in the weakly chromotryptophan-binding repressor that we want, characterized by GFP.
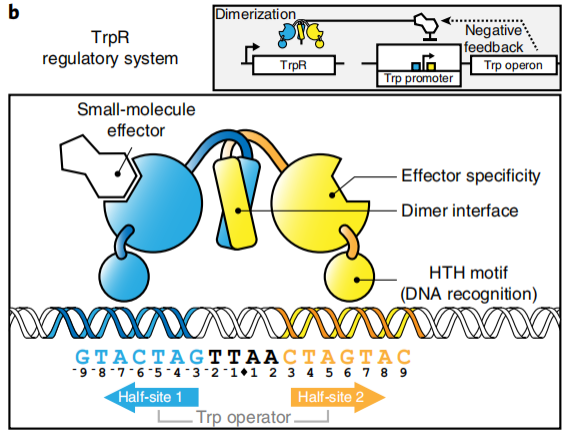
The TrpR regulatory program controls the
negative-feedback inhibition during L-tryptophan biosynthesis.



