Dry lab
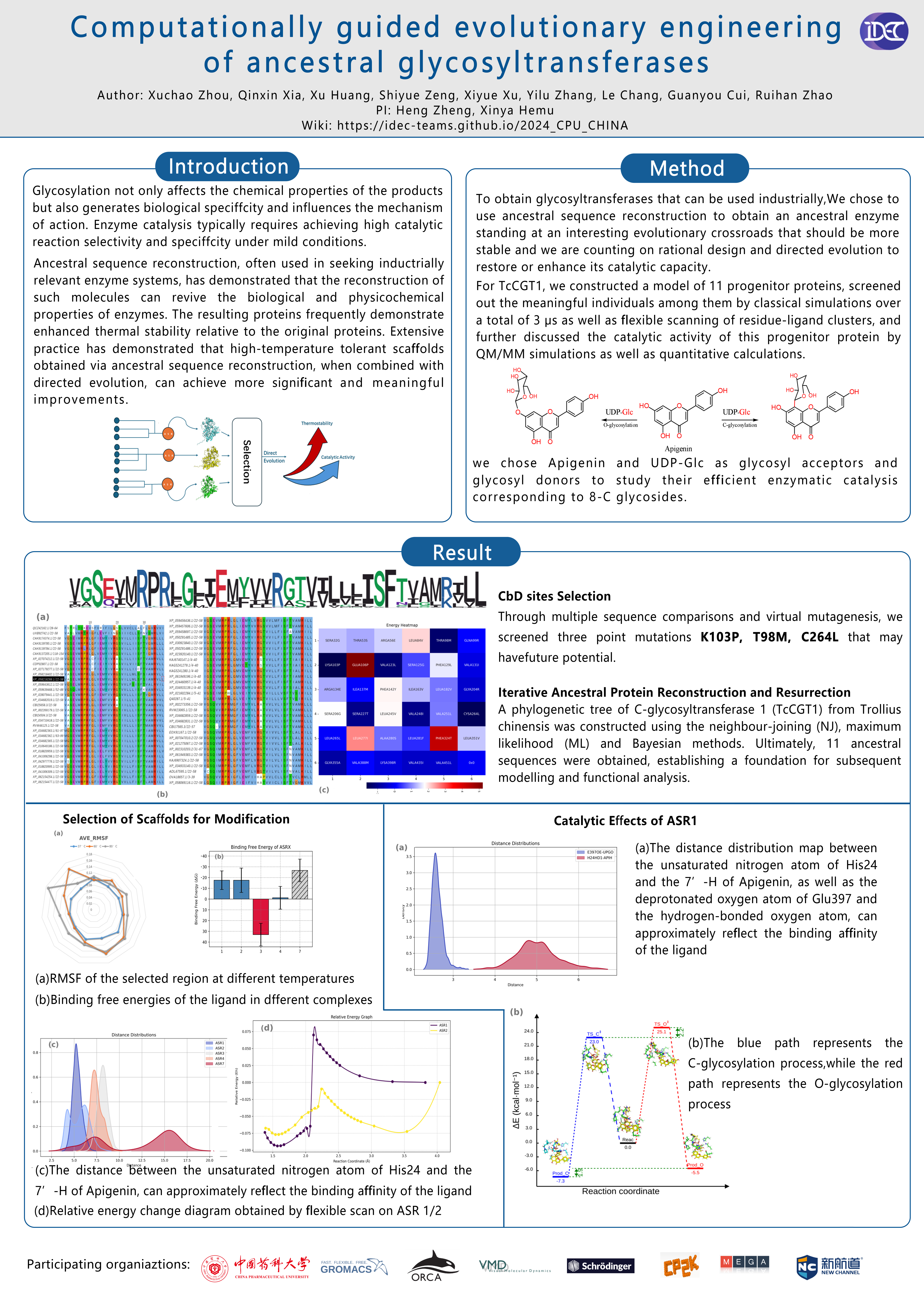
The thermal stability of biomolecules obtained by ancestral sequence reconstruction is typically higher. In order to achieve this, we reconstructed the phylogenetic tree of TcCGT1. The 11 proteins obtained through homology modeling were subjected to screening based on the AVE_RMSF of a given region at varying temperatures, binding free energy, and 8-C glycosylation energy barriers . It is anticipated that the final screened progenitor protein, ASR1, will retain some thermal stability and catalytic activity.
To investigate the catalytic ability of ASR1, the C/O-glycosylation barriers were calculated at the DFT level. Additionally, the results of QM/MM simulations suggest that ASR1 may exhibit similarities with TcCGT1.
Wet lab
For the sequences obtained after iterative ASR, we constructed plasmids and screened the novel proteases through heterologous expression in E. coli vectors, and we put the purified proteins into in vitro reactions and measured their reactivity under different environments (pH, temperature, etc.) to evaluate the strengths and weaknesses of the proteins, and together with the predicted favourable mutation sites in the previous stage, we can modify the proteins twice through targeted mutagenesis to obtain the the desired protein